Abstract
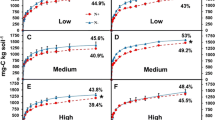
Recent evidence suggests that atmospheric nitrate (NO −3 ) deposition can alter soil carbon (C) storage by directly affecting the activity of lignin-degrading soil fungi. In a laboratory experiment, we studied the direct influence of increasing soil NO −3 concentration on microbial C cycling in three different ecosystems: black oak–white oak (BOWO), sugar maple–red oak (SMRO), and sugar maple–basswood (SMBW). These ecosystems span a broad range of litter biochemistry and recalcitrance; the BOWO ecosystem contains the highest litter lignin content, SMRO had intermediate lignin content, and SMBW leaf litter has the lowest lignin content. We hypothesized that increasing soil solution NO −3 would reduce lignolytic activity in the BOWO ecosystem, due to a high abundance of white-rot fungi and lignin-rich leaf litter. Due to the low lignin content of litter in the SMBW, we further reasoned that the NO −3 repression of lignolytic activity would be less dramatic due to a lower relative abundance of white-rot basidiomycetes; the response in the SMRO ecosystem should be intermediate. We increased soil solution NO −3 concentrations in a 73-day laboratory incubation and measured microbial respiration and soil solution dissolved organic carbon (DOC) and phenolics concentrations. At the end of the incubation, we measured the activity of β-glucosidase, N-acetyl-glucosaminidase, phenol oxidase, and peroxidase, which are extracellular enzymes involved with cellulose and lignin degradation. We quantified the fungal biomass, and we also used fungal ribosomal intergenic spacer analysis (RISA) to gain insight into fungal community composition. In the BOWO ecosystem, increasing NO −3 significantly decreased oxidative enzyme activities (−30% to −54%) and increased DOC (+32% upper limit) and phenolic (+77% upper limit) concentrations. In the SMRO ecosystem, we observed a significant decrease in phenol oxidase activity (−73% lower limit) and an increase in soluble phenolic concentrations (+57% upper limit) in response to increasing NO −3 in soil solution, but there was no significant change in DOC concentration. In contrast to these patterns, increasing soil solution NO −3 in the SMBW soil resulted in significantly greater phenol oxidase activity (+700% upper limit) and a trend toward lower DOC production (−52% lower limit). Nitrate concentration had no effect on microbial respiration or β-glucosidase or N-acetyl-glucosaminidase activities. Fungal abundance and basidiomycete diversity tended to be highest in the BOWO soil and lowest in the SMBW, but neither displayed a consistent response to NO −3 additions. Taken together, our results demonstrate that oxidative enzyme production by microbial communities responds directly to NO −3 deposition, controlling extracellular enzyme activity and DOC flux. The regulation of oxidative enzymes by different microbial communities in response to NO −3 deposition highlights the fact that the composition and function of soil microbial communities directly control ecosystem-level responses to environmental change.





Similar content being viewed by others
References
Bardner MJ, Crawford DL. 1981. Effects of carbon and nitrogen supplementation on lignin and cellulose decomposition by Streptomyces. Can J Microbiol 27:859–63
Berg B, Matzner E. 1997. Effect of N deposition on decomposition of plant litter and soil organic matter in forest systems. Environ Rev 5:1–25
Bowden RD, Davidson E, Savage K, Arabia C, Steudler P. 2004. Chronic nitrogen additions reduce total soil respiration and microbial respiration in temperate forest soils at the Harvard Forest. For Ecol Manage 196:3–56
Burton AJ, Pregitzer KS, Crawford JN, Zogg GP, Zak DR. 2004. Simulated chronic NO −3 deposition reduces soil respiration in northern hardwood forests. Global Change Biol 10:1080–91
Butnor JR, Johnsen KH, Oren R, Katul GG. 2003. Reduction of forest floor respiration by fertilization on both carbon dioxide-enriched and reference 17-year-old loblolly pine stands. Global Change Biol 9:849–61
Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst DF. 2000. Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81:2359–65
Entry JA, Backman CB. 1995. Influence of carbon and nitrogen on cellulose and lignin degradation in forest soils. Can J For Res 25:1231–6
Fog K. 1988. The effect of added nitrogen on the rate of decomposition of organic matter. Biol Rev 63:433–62.
Galloway J, Cowling E. 2002. Reactive nitrogen and the world: 200 years of change. Ambio 31:64–71
Guggenberger G. 1994. Acidification effects on dissolved organic matter mobility in spruce forest ecosystems. Enviro Int 20:31–41
Hammel KE. 1997. Fungal degradation of lignin. In: Cadish G, Giller KE, editors. Driven by nature: plant litter quality and decomposition. Wallingford (UK): CAB International. p 33–45
Henriksen TM, Breland TA. 1999. Nitrogen availability effects on carbon mineralization, fungal and bacterial growth, and enzyme activities during decomposition of wheat straw in soil. Soil Biol Biochem 31:1121–34
Hobbie SE. 2000. Interactions between litter lignin and soil nitrogen availability during leaf litter decomposition in a Hawaiian montane forest. Ecosystems 3:484–94
Homann PS, Caldwell BA, Chappell HN, Sollins P, Swanston CW. 2001. Douglas-fir soil C and N properties a decade after termination of urea fertilization. Can J For Res 31:2225–36
Johnson D, Leake JR, Lee JA, Campbell CD. 1998. Changes in soil microbial biomass and microbial activities in response to 7 years simulated pollutant nitrogen deposition on a heathland and two grasslands. Environ Pollut 103:239–50
Kalbitz K, Solinger S, Park JH, Michalzik B, Matzner E. 2000. Controls on the dynamics of dissolved organic matter in soils: a review. Soil Sci 165:277–304
Keyser P, Kirk TK, Zeikus IG. 1978. Ligninolytic enzyme of Phanerochaete chrysosporium: synthesized in the absence of lignin in response to nitrogen starvation. J Bacteriol 135: 790–7
Lopez-Llorca LV, Olivares-Bernabeu C. 1997. Growth inhibition of nematophagous and entomopathogenic fungi by leaf litter and soil containing phenols. Mycolog Res 101:691–7
Magill AH, Aber JD, Hendricks JJ, Bowden RD, Melillo JM, Steudler PA. 1997. Biogeochemical response of forest ecosystems to simulated chronic nitrogen deposition. Ecol Appl 7:402–15
Mansson KF, Falkengren-Grerup U. 2003. The effect of nitrogen deposition on nitrification, carbon and nitrogen mineralisation and litter C:N ratios in oak (Quercus robur l.) forests. For Ecol Manage 179:455–67.
McCune B, Mefford MJ. 1999. PC-ORD. Multivariate analysis of ecological data, ver. 4. Gleneden Beach (OR): MjM Software Design. 237 p
McCune B, Grace JB. 2002. Analysis of ecological communities. Gleneden Beach (OR): MjM Software Design. 300 p
McDowell WH, Currie WS, Aber JD, Yano Y. 1998. Effects of chronic nitrogen amendments on production of dissolved organic carbon and nitrogen in forest soils. Water Air Soil Pollut 105:175–82
Nadelhoffer KJ, Emmett BA, Gundersen P, Kjonaas OJ, Koopmans CJ, Schleppi P, Tietema A, others. 1999. Nitrogen deposition makes a minor contribution to carbon sequestration in temperate forests. Nature 398:145–8
Nesme X, Normand P. 2004. Easy individual strain and community typing by rDNA ITS1 analysis. In: Kowalchuk GA, Bruijn FJ, Head IA, Akkermans ADL, van Elsas JD, editors. Molecular microbial ecology manual. London: Kluwer. p 671–88
Ohno T, First PR. 1998. Assessment of the Folin and Ciacalteu’s method for determining soil phenol carbon. J Environ Qual 27:776–82
Park JH, Kalbitz K, Matzner E. 2002. Resource control on the production of dissolved organic carbon and nitrogen in a deciduous forest floor. Soil Biol Biochem 34:813–22
Pregitzer KS, Zak DR, Burton AJ, Ashby JA, MacDonald NW. 2004. Chronic nitrate additions dramatically increase the export of carbon and nitrogen from northern hardwood ecosystems. Biogeochemistry 68:179–97
Recous S, Robin D, Darwis D, Mary B. 1995. Soil inorganic n availability: effect on maize residue decomposition. Soil Biol Biochem 27:1529–38.
Sjöberg G, Bergkvist B, Berggren D, Nilsson SI. 2003. Long-term N addition effects on the C mineralization and DOC production in mor humus under spruce. Soil Biol Biochem 35:1305–15.
Waldrop MP, Zak DR, Sinsabaugh RL. 2004a. Microbial community response to nitrogen deposition in northern forest ecosystems. Soil Biol Biochem 36:1443–51
Waldrop MP, Zak DR, Sinsabaugh RL, Gallo M, Lauber C. 2004b. Nitrogen deposition modifies soil carbon storage through changes in microbial enzymatic activity. Ecol Appl 14:1172–7
White DC, Ringelberg DB. 1998. Signature lipid biomarker analysis. In: Burlage RS, Atlas R, Stahl D, Geesey G, Sayler G, editors. Techniques in microbial ecology. New York: Oxford University Press. p 255–72
Worrall JJ, Anagnost SE, Zabel RA. 1997. Comparison of wood decay among diverse lignicolous fungi. Mycologia 89:199–219
Yano Y, McDowell WH, Aber JD. 2000. Biodegradable dissolved organic carbon in forest soil solution and effects of chronic nitrogen deposition. Soil Biol Biochem 32:1743–51
Zak DR, Pregitzer KS. 1986. Spatial and temporal variability of nitrogen cycling in northern lower Michigan. For Sci 36:367–80.
Acknowledgments
This research was made possible by a grant from the Department of Energy Terrestrial Carbon Program. We thank Chris Lauber, Jana Gastellum, and Kurt Smemo for lab assistance and helpful discussion of the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Waldrop, M.P., Zak, D.R. Response of Oxidative Enzyme Activities to Nitrogen Deposition Affects Soil Concentrations of Dissolved Organic Carbon. Ecosystems 9, 921–933 (2006). https://doi.org/10.1007/s10021-004-0149-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-004-0149-0