Abstract
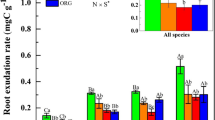
The availability of phosphorus (P) can limit net primary production (NPP) in tropical rainforests growing on highly weathered soils. Although it is well known that plant roots release organic acids to acquire P from P-deficient soils, the importance of organic acid exudation in P-limited tropical rainforests has rarely been verified. Study sites were located in two tropical montane rainforests (a P-deficient older soil and a P-rich younger soil) and a tropical lowland rainforest on Mt. Kinabalu, Borneo to analyze environmental control of organic acid exudation with respect to soil P availability, tree genus, and NPP. We quantified root exudation of oxalic, citric, and malic acids using in situ methods in which live fine roots were placed in syringes containing nutrient solution. Exudation rates of organic acids were greatest in the P-deficient soil in the tropical montane rainforest. The carbon (C) fluxes of organic acid exudation in the P-deficient soil (0.7 mol C m−2 month−1) represented 16.6% of the aboveground NPP, which was greater than those in the P-rich soil (3.1%) and in the lowland rainforest (4.7%), which exhibited higher NPP. The exudation rates of organic acids increased with increasing root surface area and tip number. A shift in vegetation composition toward dominance by tree species exhibiting a larger root surface area might contribute to the higher organic acid exudation observed in P-deficient soil. Our results quantitatively showed that tree roots can release greater quantities of organic acids in response to P deficiency in tropical rainforests.






Similar content being viewed by others
References
Ahonen-Jonnarth U, Van Hees PAW, Lundström US, Finlay RD. 2000. Production of organic acids by mycorrhizal and non-mycorrhizal Pinus sylvestris L. seedlings exposed to elevated concentrations of aluminium and heavy metals. New Phytol 146:557–67.
Aiba S, Kitayama K. 1999. Structure composition and species diversity in an altitude-substrate matrix of rain forest tree communities on Mount Kinabalu, Borneo. Plant Ecol 140:139–57.
Aiba S, Kitayama K, Repin R. 2002. Species composition and species-area relationships of trees in nine permanent plots in altitudinal sequences on different geological substrates of Mount Kinabalu. Sabah Parks Nat J 5:7–69.
Ashton PS. 1988. Dipterocarp biology as a window to the understanding of tropical forest structure. Annu Rev Ecol Syst 19:347–70.
Barber SA. 1995. Soil nutrient availability: a mechanistic approach. 2nd edn. New York: Wiley. pp 157–78.
Clark DA, Brown S, Kicklighter DW, Chamber JQ, Thomlinson JR, Ni J, Holland EA. 2001. Net primary production in tropical forests: an evaluation and synthesis of existing field data. Ecol Appl 11:371–84.
Fujii K, Hayakawa C, Van Hees PAW, Funakawa S, Kosaki T. 2010. Biodegradation of low molecular weight organic compounds and their contribution to heterotrophic soil respiration in three Japanese forest soils. Plant Soil 334:475–89.
Fujii K, Aoki M, Kitayama K. 2012. Biodegradation of low molecular weight organic acids in rhizosphere soils from a tropical montane rain forest. Soil Biol Biochem 47:142–8.
Gardner WK, Barber DA, Parbery DG. 1983. The acquisition of phosphorus by Lupinus albus L. III. The probable mechanism by which phosphorus movement in the soil/root interface is enhanced. Plant Soil 70:107–24.
Giesler R, Högberg MN, Strobel BW, Richter A, Nordgren A, Högberg P. 2007. Production of dissolved organic carbon and low-molecular weight organic acids in soil solution driven by recent tree photosynthate. Biogeochemistry 84:1–12.
Gimaret-Carpentier C, Dray S, Pascal JP. 2003. Broad-scale biodiversity pattern of the endemic tree flora of the Western Ghats (India) using canonical correlation analysis of herbarium records. Ecography 26:429–44.
Grayston SJ, Vaughan D, Jones D. 1996. Rhizosphere carbon flow in trees, in comparison with annual plants: the importance of root exudation and its impact on microbial activity and nutrient availability. Appl Soil Ecol 5:29–56.
Inagaki M, Inagaki M, Kamo K, Miyamoto K, Titin J, Jamalung L, Lapongan J, Miura S. 2011. Nitrogen and phosphorus retranslocation and N:P ratios of litterfall in three tropical plantations: luxurious N and efficient P use by Acacia mangium. Plant Soil 341:295–307.
Jacobson G. 1970. Gunung Kinabalu Area, Sabah, Malaysia. Geological Survey Malaysia, Report 8. Kuching (Malaysia): Geological Survey Malaysia.
Jones DL. 1998. Organic acids in the rhizosphere—a critical review. Plant Soil 205:25–44.
Jones DL, Hodge A, Kuzyakov Y. 2004. Plant and mycorrhizal regulation of rhizodeposition. New Phytol 163:459–80.
Jordan CF, Herrera R. 1981. Tropical rain forests: are nutrients really critical? Am Nat 117:167–80.
Kitayama K. 2005. Comments on “Ecosystem properties and forest decline in contrasting long-term chronosequences”. Science 308:633.
Kitayama K, Aiba S. 2002. Ecosystem structure and productivity of tropical rain forests along altitudinal gradients with contrasting soil phosphorus pools on Mount Kinabalu, Borneo. J Ecol 90:37–51.
Kitayama K, Majalap-Lee N, Aiba S. 2000. Soil phosphorus fractionation and phosphorus-use efficiencies of tropical rainforests along altitudinal gradients of Mount Kinabalu, Borneo. Oecologia 123:342–9.
Kitayama K, Aiba S, Takyu M, Majalap N, Wagai R. 2004. Soil phosphorus fractionation and phosphorus-use efficiency of a Bornean tropical montane rain forest during soil aging with podozolization. Ecosystems 7:259–74.
Kitayama K, Aiba S, Ushio M, Seino T, Fujiki Y. 2011. The ecology of Podocarps in tropical montane forests of Borneo: distribution, population dynamics, and soil nutrient acquisition. In: Turner BL, Cernusak LA, Eds. Ecology of the Podocarpaceae in tropical forests. Smithsonian Contributions to Botany, No. 95. Washington, DC: Smithsonian Institution Scholarly Press. pp 101–17.
Lambers H, Raven JA, Shaver GR, Smith SE. 2008. Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23:95–103.
Landeweert R, Hoffland E, Finlay RD, Kuyper TW, Van Breemen N. 2001. Linking plants to rocks: ectomycorrhizal fungi mobilize nutrients from minerals. Trends Ecol Evol 16:248–54.
Liu Q, Loganathan P, Hedley MJ, Skinner MF. 2006. Root processes influencing phosphorus availability in volcanic soils under young Pinus radiata plantations. Can J For Res 36:1913–20.
McKeague JA, Day JH. 1966. Dithionite- and oxalate-extractable Fe and Al as aids in differentiating various classes of soils. Can J Soil Sci 46:13–22.
Naik D, Smith E, Cumming JR. 2009. Rhizosphere carbon deposition, oxidative stress and nutritional changes in two popular species exposed to aluminum. Tree Physiol 29:423–36.
Neumann G, Massonneau A, Langlade N, Dinkelaker B, Hengeler C, Römheld V, Martinoia E. 2000. Physiological aspects of cluster root function and development in phosphorus-deficient white lupin (Lupinus albus L.). Ann Bot 85:909–19.
Ostertag R. 2001. Effects of nitrogen and phosphorus availability on fine-root dynamics in Hawaiian montane forests. Ecology 82:485–99.
Phillips RP, Erlitz Y, Bier R, Bernhardt ES. 2008. New approach for capturing soluble root exudates in forest soils. Funct Ecol 22:990–9.
Powers JS, Treseder KK, Lerdau MT. 2005. Fine roots, arbuscular mycorrhizal hyphae and soil nutrients in four neotropical rain forests: patterns across large geographic distances. New Phytol 165:913–21.
Richardson SJ, Peltzer DA, Allen RB, McGlone MS, Parfitt RL. 2004. Rapid development of phosphorus limitation in temperate rainforest along the Franz Josef soil chronosequence. Oecologia 139:267–76.
Smith WH. 1970. Root exudates of seedling and mature sugar maple. Phytopathology 60:701–3.
Soil Survey Staff. 2006. Keys to soil taxonomy. 10th edn. Washington, DC: United States Department of Agriculture Natural Resources Conservation Service.
Sollins P. 1998. Factors influencing species composition in tropical lowland rain forest: does soil matter? Ecology 79:23–30.
Ström L, Olsson T, Tyler G. 1994. Differences between calcifuges and acidifuge plants in root exudation of low-molecular organic acids. Plant Soil 167:239–45.
Ström L, Owen AG, Godbold DL, Jones DL. 2002. Organic acid mediated P mobilization in the rhizosphere and uptake by maize roots. Soil Biol Biochem 34:703–10.
Tiessen H, Moir JO. 1993. Characterization of available P by sequential extraction. In: Carter MR, Ed. Soil sampling and methods of analysis. Boca Raton, FL: Lewis Publishers. pp 75–86.
Treseder KT, Vitousek PM. 2001. Effects of soil nutrient availability on investment in acquisition of N and P in Hawaiian rain forests. Ecology 82:946–54.
Tuason MMS, Arocena M. 2009. Root organic acid exudates and properties of rhizosphere soils of white spruce (Picea glauca) and subalpine fir (Abies lasiocarpa). Can J Soil Sci 89:287–300.
Turner BL, Condron LM, Richardson SJ, Peltzer DA, Allison VJ. 2007. Soil organic phosphorus transformations during pedogenesis. Ecosystems 10:1166–81.
Uselman SM, Qualls RG, Thomas RB. 1999. A test of a potential short cut in the nitrogen cycle: the role of exudation of symbiotically fixed nitrogen from the roots of a N-fixing tree and the effects of increased atmospheric CO2 and temperature. Plant Soil 210:21–32.
Van Hees PAW, Dahlen J, Lundström US, Boren H, Allard B. 1999. Determination of low molecular weight organic acids in soil solution by HPLC. Talanta 48:173–9.
Van Hees PAW, Jones DL, Finlay R, Godbold DL, Lundström US. 2005. The carbon we do not see-the impact of low molecular weight compounds on carbon dynamics and respiration in forest soils: a review. Soil Biol Biochem 37:1–13.
Van Schöll L, Hoffland E, Van Breemen N. 2006. Organic anion exudation by ectomycorrhizal fungi and Pinus sylvestris in response to nutrient deficiencies. New Phytol 170:153–63.
Walker TW, Syers JK. 1976. The fate of phosphorus during pedogenesis. Geoderma 15:1–19.
Wardle DA, Walker LR, Bardgett RD. 2004. Ecosystem properties and forest decline in contrasting long-term chronosequences. Science 305:509–12.
Acknowledgments
We thank the editor and anonymous reviewers for their helpful suggestions and comments on the manuscript. We are grateful to S. Aiba, R. Aoyagi, and M. Nagaoka for their assistance with field work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
Conceived of or designed study: M. Aoki, K. Fujii, K. Kitayama; performed research: M. Aoki, K. Fujii, K. Kitayama; analyzed data: M. Aoki, K. Fujii; contributed new methods or models: K. Fujii; wrote the paper: K. Fujii, M. Aoki, K. Kitayama.
Rights and permissions
About this article
Cite this article
Aoki, M., Fujii, K. & Kitayama, K. Environmental Control of Root Exudation of Low-Molecular Weight Organic Acids in Tropical Rainforests. Ecosystems 15, 1194–1203 (2012). https://doi.org/10.1007/s10021-012-9575-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-012-9575-6