Abstract
In several mammalian and avian species, females show a higher performance than males in tasks requiring cognitive flexibility such as the discrimination reversal learning. A recent study showed that female guppies are twice as efficient as males in a reversal learning task involving yellow–red discrimination, suggesting a higher cognitive flexibility in female guppies. However, the possibility exists that the superior performance exhibited by females does not reflect a general sex difference in cognitive abilities, but instead, is confined to colour discrimination tasks. To address this issue, we compared male and female guppies in two different discrimination reversal learning tasks and we performed a meta-analysis of these experiments and the previous one involving colour discrimination. In the first experiment of this study, guppies were tested in a task requiring them to learn to select the correct arm of a T-maze in order to rejoin a group of conspecifics. In experiment 2, guppies were observed in a numerical task requiring them to discriminate between 5 and 10 dots in order to obtain a food reward. Although females outperformed males in one condition of the T-maze, we did not find any clear evidence of females’ greater reversal learning performance in either experiment. However, the meta-analysis of the three experiments supported the hypothesis of females’ greater reversal learning ability. Our data do not completely exclude the idea that female guppies have a generally higher cognitive flexibility than males; however, they suggest that the size of this sex difference might depend on the task.
Similar content being viewed by others
Introduction
Among several polygamous mammals and birds, females show greater cognitive flexibility and reduced persistence compared to males (Guillamón et al. 1986; Ha et al. 2011; Roelofs et al. 2017; Rogers 1974). This sex difference is often studied with the discrimination reversal learning task: the animal is initially trained to choose a predetermined stimulus between two alternative options to obtain a reward; once having learned the association, the reward contingency is reversed and the speed in learning the new association is taken as a measure of flexibility (Shettleworth 2010).
The proximate mechanisms underlying sex differences in cognitive flexibility are likely hormonal: for example, in rats, administering androgens to females and castrating males reverses the direction of the sex difference (Guillamón et al. 1986). The evolutionary explanation of this sex difference is instead less clear. A greater reversal learning ability in females is usually absent in bird species with a monogamous mating system (Brust et al. 2013; Titulaer et al. 2012). Thus, this sex difference might have evolved because of the different selective pressures acting on the two sexes in polygamous species. In line with this hypothesis, greater male persistence has been supposed to evolve in order to overcome female resistance to mate (Rowe et al. 2005).
Recently, one study found evidence of a better performance of females in discrimination reversal learning in a polygamous fish species, the guppy Poecilia reticulata (Lucon-Xiccato and Bisazza 2014). Guppies were trained to dislodge small discs on the bottom of their testing tank to obtain food concealed underneath. Discs had two colours, red and yellow, but only one predetermined colour hid the food. Both males and females quickly learned to associate the presence of food with the rewarded colour, but when the reinforced colouration was reversed, females learned the new condition much faster than males, which instead continued to persist on the previously reinforced colour. This result might indicate that polygamy favours the evolution of greater female flexibility and greater male persistence in fish too. However, before accepting this hypothesis, an alternative explanation should be considered. In the aforementioned study, guppies were tested in a yellow–red discrimination and the possibility exists that the different performance is related to the different ecological relevance of colour discrimination for the two sexes.
Female guppies typically choose among available mates based on the size, number and pattern of male carotenoid orange body spots (Houde 1997). Several studies highlighted an astonishing ability of female guppies to learn the colour pattern of males after a single encounter and use this information in future mating decisions (Eakley and Houde 2004; Hughes et al. 1999). Furthermore, female guppies are known to be extremely flexible in their mating decisions. For example, the preference of a focal female for one specific male over another one can be easily reversed if the focal female could observe the non-preferred male mating with other females (Dugatkin and Godin 1992; Godin et al. 2005). Reversal of female mate preference has been observed also when the focal female is exposed to predation risk (Gong and Gibson 1996).
Colour discrimination has a very important role for guppies in the foraging context too. Both males and females have a strong tendency to search for small carotenoid-rich fruits that drop from the forest canopy into the rivers (Rodd et al. 2002). In the laboratory, yellow–red discrimination occurs incredibly faster than other types of discrimination (Lucon-Xiccato and Bisazza 2014, 2016). Since dietary requirements could be different in the two sexes (e.g. because carotenoids affect mating success in males but not in females; Grether 2000; Rodd et al. 2002), natural selection might have shaped discrimination learning mechanisms differently in the two sexes.
To clarify this issue, we need to assess whether a greater female cognitive flexibility is observed in other contexts and, in case, if the magnitude of this difference is comparable to colour discrimination learning. In this study, we performed two reversal learning experiments not involving colour discrimination. In experiment 1, the task consisted of a T-maze, which is usually adopted in literature to study spatial discrimination reversal learning (Elias et al. 1973; Watson and Stanton 2009). In experiment 2, the task was a discrimination between two sets of dots differing in numerosity. Both spatial and numerical abilities have been widely investigated in guppies (Agrillo et al. 2017; Kellogg and Gavin 1960; Lucon-Xiccato and Bisazza 2017a). If a general sex difference in cognitive flexibility exists in guppies, females are expected to outperform males in both the discrimination reversal learning tasks. On the contrary, a lack of differences between the two sexes might imply that the previously observed sex difference in flexibility (Lucon-Xiccato and Bisazza 2014) was related to the specific context of colour discrimination.
Materials and methods
Subjects
We used guppies of the same strain and age and the same sample size of the previous study (Lucon-Xiccato and Bisazza 2014). These guppies derived from an outbred aquarium stock (snakeskin cobra green) bred in our laboratory since 2012. In experiment 1, we used 14 males and 14 females, but one female stopped participating after the first learning phase; thus, for this experiment the final sample consisted of 13 females. In experiment 2, we used 40 guppies (20 males and 20 females). A total of 12 subjects were excluded from this experiment. In detail, two males were excluded because they did not complete the pre-training, two males and three females ceased to participate during the numerical discrimination phase, and one male and four females ceased to participate during the reversal learning phase (see “Procedure” of experiment 2). As a consequence, the total sample consisted of 14 males and 14 females, with a proportion of fish (70%) successfully completing the experiment similar to that reported in previous studies that used this operant conditioning procedure (Lucon-Xiccato and Bisazza 2014, 2016; Miletto Petrazzini et al. 2015b).
Before the experiments, guppies were maintained in 150-l tanks with natural gravel bottom, vegetation (Hygrophila corymbosa and Taxiphyllum barbieri) and water filters. Water temperature was kept at 26 ± 1 °C. Fluorescent lamps illuminated the tanks with a 12:12-h light/dark cycle. We fed guppies 3 times per day using commercial food flakes and live Artemia salina nauplii.
Experiment 1: T-maze reversal learning
We used a procedure previously adopted to study spatial learning abilities in guppies (Lucon-Xiccato and Bisazza 2017b). Guppies were required to choose the correct arm of a T-maze to reach their home environment in which a group of social companions was present. As far as possible, we mirrored the procedure adopted in the first study on sex differences in reversal learning in the guppy (Lucon-Xiccato and Bisazza 2014).
Apparatus
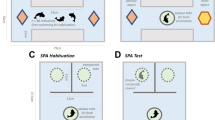
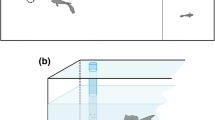
The experimental apparatus consisted of a T-maze inserted in a 68 × 68 × 35 cm glass tank filled with 25 cm of water (Fig. 1a). The tank was inhabited by two subjects and by twenty-five immature guppies that served as social companions during the experiment (see “Procedure”). This part of the tank resembled the maintenance tanks as it was provided with natural gravel, vegetation (H. corymbosa and T. barbieri) and water filters. We turned off water filters during the trials to prevent the flow of water from affecting subjects’ behaviour in the maze.
Experimental apparatuses. a Experiment 1: the experimental apparatus was a T-maze inserted in a tank filled with water. The maze consisted of a starting chamber, a central arm and two identical lateral arms connected to the external tank. The terminal part of the arms was S shaped to prevent the subjects from seeing the exit from inside the maze, b experiment 2: the apparatus was divided into the back compartment and the front experimental compartment. The start box was inserted between the compartments. Stimuli consisted of arrays of black dots on a white background placed orthogonally to the green panel in the experimental compartment. The holes in front of each stimulus were completely covered with a pair of yellow discs (colour figure online)
The maze was placed in the middle of the tank on a plastic support that kept it 2 cm below water level. The maze was made of green plastic panels and consisted of a starting chamber (8 × 8 cm), one 15-cm central arm and two identical 8-cm lateral arms. The width of the three arms was 3 cm because it was found that small corridors motivate guppies to exit a maze (Kellogg and Gavin 1960). The two lateral arms were both connected to the external tank, but the terminal part of these arms was S shaped to prevent the subjects from seeing the exit from inside the maze. To avoid latent learning of the maze (Gómez-Laplaza and Gerlai 2010), a plastic panel occluded the exit of the maze outside the trials and prevented the entrance of the subjects and the social companions. During the trials, we placed a grid net just before each exit. The grid in the correct arm presented a small hole to allow the subject to access the external tank, whereas the grid in the wrong arm did not present the hole, preventing the subject from exiting. By changing the two grids, we could modify which arm was the correct one that guppies should use to reach the external tank. The apparatus was placed in a dark room and surrounded by black curtains to prevent the fish from seeing the chamber. The maze was built symmetrically with no landmarks. Thus, there were no additional internal or external visual cues available for the subjects to solve the task. One camera placed over the maze recorded the trials.
Procedure
We moved two subjects, one male and one female, from the maintenance tank to the external part of the experimental tank 24 h before the start of the experiment. We administered six training trials daily, divided into two three-trial sessions (one in the morning and one in the afternoon). During each trial, we gently moved one subject into the starting chamber. We alternated the order of males and females between trials. The subject was free to swim in the maze and find the correct arm. For half of the subjects, the correct arm during the initial discrimination learning was the right arm, and for the remaining subjects, the correct arm was the left one. The experimenter took note of whether the subject entered first the correct or the incorrect arm. When the subject performed eight correct out of ten consecutive trials, it was considered to have learned the discrimination; thus, in the following session, we switched the correct arm and trained the fish with the reversed contingency to the same learning criterion. Since fish underwent six trials per day (in two sessions of three trials each), the criterion could be reached by cumulating the trials from 2 or 3 consecutive days.
Experiment 2: Numerical reversal learning
In this experiment, we compared male and female guppies in a numerical task using a reversal learning paradigm similar to that previously adopted by Lucon-Xiccato and Bisazza (2014). Guppies were initially trained to discriminate between two numerosities (5 vs. 10 dots). After reaching the learning criterion, the reward contingency was reversed.
Apparatus and stimuli
We used the apparatus previously adopted to study numerical abilities in guppies (Miletto Petrazzini et al. 2015a, b). It consisted of a glass tank (60 × 40 × 35 cm) filled with gravel and 30 cm of water maintained at a temperature of 26 ± 1 °C (Fig. 1b). Green opaque partitions divided the apparatus into a back compartment provided with natural vegetation and a front “experimental compartment.” A start box was inserted between the compartments and was provided with a transparent guillotine door controlled remotely by the experimenter. The apparatus was provided with two filters, and each compartment was lit by a 15-W fluorescent light. A green plastic panel (20 × 15 cm) with 46 holes (Ø 1 cm, depth 0.5 cm) was placed in the experimental compartment, close to the front wall. A green net covered the bottom of the panel to allow the smell of the food reward to pervade the whole compartment in order to reduce the possibility of the subject using olfactory cues to locate the correct choice. Two yellow plastic discs (Ø 1.2 cm, height 0.2 cm) were used during the experiment to cover the holes of the panel in front of the stimuli. In order to avoid social isolation of the subject, four social companions were kept in the apparatus and were temporarily removed before each experimental session.
Stimuli consisted of pairs of laminated panels placed orthogonally to the green panel. Stimuli were groups of black dots on a white background (5 × 5 cm). Only one numerical contrast was presented during the experiment: 5 versus 10 (0.50 ratio). Numerosity normally co-varies with several physical attributes (e.g. cumulative surface area, overall space occupied by the sets or density of the elements), commonly called “continuous quantities,” that can be used to estimate which group is larger/smaller (Feigenson et al. 2002; Pisa and Agrillo 2009; Gebuis and Reynvoet 2012). In order to reduce the possibility of subjects using these non-numerical cues to solve the task, the stimuli were controlled for continuous quantities. In particular, in one-third of the stimuli, the cumulative surface area was matched to 100%. However, a by-product of equating the cumulative surface area was that smaller-than-average items would be more frequent in the larger groups, and guppies might use this information instead of number. To reduce this possibility, the cumulative surface area was matched to 75% in another third of the stimuli and in the remaining third, the cumulative surface area was not controlled (i.e. the ratio between the cumulative surface area within each pair was congruent with the numerical ratio: 0.5). Furthermore, since density and convex hull (that is, the overall surface encompassed by the most lateral dots) are negatively correlated, half of the sets was controlled for the convex hull, whereas the second half was controlled for density. Stimuli were extracted from a pool of 48 different pairs (16 pairs for each area control). The spatial distribution of the dots and their size varied across stimuli to avoid the possibility that fish could have learned the discrimination on the basis of canonical pattern recognition instead of numerical information (Mandler and Shebo 1982). Provided that no other non-numerical cue could be used to solve the task, subjects could reach the learning criterion only if they were sensitive to numerosity (reviewed in Agrillo and Bisazza 2014).
Procedure
We used a modification of the procedures recently adopted to study numerical abilities (Miletto Petrazzini et al. 2015a) and behavioural flexibility in guppies (Lucon-Xiccato and Bisazza 2014). The procedure consisted of five phases: familiarization, pre-training, numerical discrimination learning, discrimination reversal learning and olfactory cue control test.
Familiarization
During the 5 days preceding the beginning of the pre-training phase, fish were gradually familiarized with the apparatus and the experimental procedure. On days 1–2, two subjects were introduced into the experimental apparatus and were fed with commercial food flakes released through a Pasteur pipette into the water three times a day. On days 3–5, for three times a day, fish were gently ushered into the start box using a transparent plastic panel. Once the subjects were into the start box, the guillotine door was closed and a green plastic barrier was placed in front of it to prevent the fish from seeing the experimental compartment. A pair of stimuli was placed on the far end of the green panel, and dry food was delivered into four holes in the middle of the panel and equidistant from the stimuli. Subsequently, fish were allowed to enter the experimental compartment and eat the food. Each area control (100, 75% and non-controlled) was presented once a day. On the evening of day 5, fish were individually housed in an experimental apparatus. The training phase began the next day.
Pre-training
During this phase, fish were trained to dislodge the discs. For this aim, fish underwent a total of 9 trials with partially covered holes. In trials 1–3, discs covered 25% of the holes; in trials 4–6, they covered 50%; and in trials 7–9, they covered 75% of the holes. The food reward was placed only under the disc of the positive stimulus. All subjects were presented with the same numerical contrast: 5 versus 10. However, the positive stimulus was the larger numerosity (10) for half of the fish, whereas the positive stimulus was the smaller numerosity (5) for the other half. These nine trials were excluded from the analysis. Only subjects that learned to dislodge the discs were admitted to the numerical discrimination learning phase.
Numerical discrimination learning
During this phase, the fish were subjected to six trials per day, subdivided into two sessions of three trials each with a 4-h inter‐session interval. Each trial started with the subject in the start box, the guillotine door closed and the green barrier in front of it to block the view of the experimental compartment. The experimenter placed a pair of stimuli on the green plate, hid the food reward in the hole in front of the positive stimulus and used the yellow discs to entirely cover the holes in correspondence with the stimuli. Subsequently, the barrier was removed and the fish was allowed to look at the stimuli for 30 s. After that, the door was opened and the subject could enter the experimental compartment to make its choice. The choice was defined as the first disc dislodged by the fish. We used a correction procedure to keep the subjects motivated, and if the fish opened the disc associated with the wrong stimulus, it was allowed to open the disc associated with the correct one and eat the food. If no discs were dislodged within 5 min, the trial was considered invalid and repeated later. The position of the stimuli on the plate and their distance from the corridor were determined with a pseudorandom rule. To avoid any side bias, the left–right position of the stimuli was counterbalanced over trials and the positive stimulus was never presented more than twice in a row on the same side. Each cumulative surface area control (100, 75% and non-controlled) was presented twice a day. The training continued until the subject reached a learning criterion of 8 correct responses out of 10 consecutive trials as in experiment 1. Once the criterion was reached, the fish entered the discrimination reversal learning phase.
Discrimination reversal learning
The procedure was the same one used in the numerical discrimination learning phase, but the reward contingency was reversed. The positive stimulus was 10 dots for the subjects previously trained to select 5 dots and vice versa. The trials always started in the session following the one in which the subjects had reached the criterion in the numerical discrimination task. The learning criterion was the same used in the numerical discrimination learning phase.
Olfactory control test
Subjects were given 15 trials using the same procedure of the reversal learning except that they were presented with pairs of stimuli of identical numerosity. The subjects trained to select 10 dots in the reversal learning phase were presented with 10 versus 10, whereas the subjects trained to select 5 dots in the reversal learning phase were presented with 5 versus 5. The food reward was hidden only under one disc with the assumption that, if the subjects used olfactory cues to locate the correct choice in the previous phases, they were expected to select the rewarded stimulus more than chance.
Statistical analysis
Analyses were performed in R version 3.2.2 (The R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org). In the text, we provided mean ± SD. All the statistical tests were two-tailed, and the significance threshold was set at P = 0.05. The number of errors in experiment 2 was log-transformed before the analysis because of a right-skewed distribution. For both experiments, we compared the performance in the initial discrimination learning and in the reversal learning phase. To do this, we performed repeated measures ANOVA on the pooled data of the initial and the reversal learning phase, fitted with phase (initial/reversal) as within-subject factor. To compare the performance of the two sexes in the two experimental phases, we fitted ANOVAs on the number of errors to criterion with sex as factor. We also fitted the side initially associated with the reward (right or left) and the initially rewarded numerosity (5 or 10) as factor in experiment 1 and experiment 2, respectively. We used one-sample t test to compare the choice for the baited disc in the control test of experiment 2 against chance level (50%). In case of absence of significant effect of the sex in the previous analysis, the use of the null hypothesis significance testing does not allow to conclude that the performance of males and females was similar (Barchard 2015) nor to exclude the presence of a sex difference smaller compared to that observed in the previous reversal learning study (Lakens and Evers 2014). For this reason, we calculated Cohen’s d effect sizes and their 95% CIs using the “effsize” R package. We also calculated the approximate Bayes factor (BF) in favour of the null hypothesis (i.e. the two sexes have similar performance) by comparing the Bayes information criterion of the linear model fitted with and without the term sex (Wagenmakers 2007). BF provides relative support to the null hypothesis that is robust even with reduced sample size (Jeffreys 1961). For example, a BF > 10 would conventionally be considered strong evidence for the absence of sex difference (Jeffreys 1961).
To analyse all data available on sex differences in reversal learning in guppies, including the previous study (Lucon-Xiccato and Bisazza 2014), we adopted a meta-analytic approach using the “rma” function of the “metafor” R package on the log-transformed data of the experiments (Viechtbauer 2010). One last analysis was aimed to understand whether possible differences between the present and the previous reversal learning experiment might be due to stochastic resampling of the same population. Using bootstrap, we simulated 10,000 data sets of 14 males and 14 females based on the data of the colour discrimination reversal learning (Lucon-Xiccato and Bisazza 2014) and we calculated the mean performance difference between males and females (Crawley 2012). From the distribution of the simulated mean differences and the mean differences observed in the two reversal learning experiments of this study, we calculated P values that describe the likelihood of observing the data of the present experiments due to stochastic resampling from the data of the previous experiment.
Results
Experiment 1: T-maze reversal learning
In the initial learning phase, guppies made 13.81 ± 11.96 errors before learning the correct arm of the maze. In the reversal learning phase, guppies made 14.49 ± 14.85 errors before learning the new reward contingency. The repeated measures ANOVA did not find a significant difference between the number of errors made in the two phases (F 1,26 < 0.001, P = 0.987).
The ANOVA analysing the number of errors in the initial learning phase did not find differences between males and females [males: 15.93 ± 11.94; females: 11.54 ± 12.03; F 1,23 = 2.335, P = 0.140; Cohen’s d = 0.31, 95% CI (−0.49, 1.11); Fig. 2a]. Guppies made fewer errors when the correct arm was the right one (right arm: 8.43 ± 5.81; left arm: 19.62 ± 14.25; F 1,23 = 6.959, P = 0.015). There was not significant sex × side interaction (F 1,23 = 0.212, P = 0.650).
The ANOVA analysing the number of errors in the reversal learning phase did not find differences between males and females [males: 17.79 ± 17.78; females: 11.69 ± 9.59; F 1,23 = 0.643, P = 0.431; Cohen’s d = 0.27, 95% CI (−0.53, 1.07); Fig. 2a]. There was no difference in the number of errors between guppies initially trained with the right or the left arm associated with the reward (right arm: 15.71 ± 11.25; left arm: 13.92 ± 17.78; F 1,23 = 1.038, P = 0.319). The sex × side interaction was significant in the model (F 1,23 = 4.492, P = 0.045), indicating that females performed better than males when the initial rewarded arm was the left one (Fig. 2b). The Bayesian analysis provided moderate support to the absence of sex difference (BF = 3.98).
Experiment 2: Numerical reversal learning
In the initial learning phase, guppies made 11.78 ± 9.02 errors before learning to choose the correct numerosity. In the reversal learning phase, guppies made 12.39 ± 4.55 errors before learning the new reward contingency. The repeated measures ANOVA did not find a significant difference between the number of errors made in the two phases (F 1,27 = 0.101, P = 0.753).
The ANOVA on the number of errors in the initial learning phase found that females made fewer errors than males [males: 15.14 ± 9.36; females: 8.43 ± 7.55; F 1,24 = 4.425, P = 0.046; Cohen’s d = 0.79, 95% CI (−0.02, 1.60); Fig. 3a] with no difference between fish trained to select the larger numerosity as positive and fish trained to select the smaller numerosity (respectively, 9.57 ± 8.76 and 14.00 ± 9.04; F 1,24 = 1.925, P = 0.178). There was not significant interaction between sex and the numerosity (larger vs. smaller) associated with the food reward during the training (F 1,24 = 0.451, P = 0.508).
In the reversal learning phase, the ANOVA on the number of errors did not find any significant difference, neither between the two sexes [males: 13.71 ± 3.60; females: 11.07 ± 5.12; F 1,24 = 2.330, P = 0.140; Cohen’s d = 0.60, 95% CI (−0.20, 1.39); Fig. 3b] nor between the numerosity rewarded (larger: 12.36 ± 5.60; smaller: 12.43 ± 3.41; F 1,24 = 0.002, P = 0.967). There was not significant interaction between sex and the numerosity (larger vs. smaller) associated with the food reward during the training (F 1,24 = 0.288, P = 0.597). The Bayesian analysis did not support the absence of sex difference (BF = 1.47).
In the control test, guppies selected the disc hiding the food reward at chance level (0.48 ± 0.13; t 27 = 0.875, P = 0.389), showing that they did not use olfactory cues to select the correct numerosity during the experiment.
Meta-analysis of sex differences in reversal learning in guppies
The meta-analysis on all the available data on sex differences in discrimination reversal learning found evidence of a significant females’ greater reversal learning ability (P = 0.013; Fig. 4a). The bootstrap analysis showed that the mean difference between the reversal learning performance of males and females observed in the two experiments of this study could not be obtained by resampling the data of the previous colour reversal learning experiment (experiment 1: P = 0.006; experiment 2: P = 0.028). Furthermore, the conditional probability to obtain two independent experiments giving no sex difference as in this study is significantly lower than expected based on the colour reversal learning experiment (P = 0.0002; Fig. 4b). This suggests that the difference between the present and the previous study is not due to subject random sampling.
Results of the comparison between experiments on guppies’ sex differences in reversal learning. a Mean differences (and 95% CI; logarithmic transformation) between the number of errors made by males and females in the colour discrimination reversal learning (Lucon-Xiccato and Bisazza 2014), the spatial (T-maze) and numerical discrimination reversal learning (present study) and estimated from the meta-analysis, b histogram of the mean difference between the number of errors (logarithmic transformation) made by males and females obtained with bootstrap from the data of the colour discrimination reversal learning (Lucon-Xiccato and Bisazza 2014); black, grey and dotted grey line are observed difference between males and females in the colour, spatial and numerical discrimination reversal learning, respectively
Discussion
A previous study found a large sex difference in guppies’ ability to perform a reversal learning task involving yellow–red colour discrimination, with males making about twice as many errors as females (Lucon-Xiccato and Bisazza 2014). This could indicate an overall greater cognitive flexibility of females, as observed in some mammals and birds (Guillamón et al. 1986; Ha et al. 2011; Roelofs et al. 2017; Rogers 1974). Here, we confronted male and female guppies in two other reversal learning tasks requiring T-maze and numerical discrimination, respectively. In both experiments, we found no obvious evidence of sex difference in discrimination reversal learning. The females’ greater performance emerged only when jointly analysing the present and the previous experiments on guppies’ reversal learning performance.
In the first experiment, we tested guppies in a T-maze. Although we did not assess whether the fish solved the task by learning the correct arm or developing an egocentric strategy (e.g. turn left), the T-maze performance is usually considered a measure of spatial discrimination abilities (Elias et al. 1973; Watson and Stanton 2009). Guppies needed approximately the same number of trials in the learning and reversal phases. We found no sex difference in the initial learning phase performance, but females outperformed males in one condition of the reversal learning phase (when the initial reinforced direction was the left one). Previously, male and female guppies were compared in other spatial tasks (Lucon-Xiccato and Bisazza 2017a, b). In one experiment, males and females showed similar learning abilities when tested in a simple spatial task, detouring around a semi-transparent barrier to reach a social group. In another test, consisting in learning the correct door in two sequential pairs of binary choices, males outperformed females. An opposite sex difference was observed in a task consisting of learning a complex maze made of six consecutive T-junctions. In the initial discrimination of experiment 1, we found that reinforced direction had a very strong effect, with both males and females making more errors when the correct choice was a left turn. The same bias was found in a previous study using the complex maze formed by six sequential T-junctions (Lucon-Xiccato and Bisazza 2017b). As previously observed in guppies and other poeciliid fish, the most likely explanation for this turning bias is an effect of cerebral lateralization (Bisazza and Brown 2011).
The second experiment followed the same procedure as the previous colour discrimination reversal learning experiment (Lucon-Xiccato and Bisazza 2014), but here, guppies were trained in a 5 versus 10 numerical discrimination with stimuli controlled for non-numerical cues (cumulative surface area, density and convex hull). We found that females were significantly better than males at learning this discrimination, whereas we did not find significant sex differences in the reversal phase. This result seems to suggest that females have better numerical abilities than males. However, this hypothesis must be considered with caution. Two previous studies did not find sex differences in two diverse numerical tasks, which were a discrimination of food quantities and a discrimination of ordinal positions (Lucon-Xiccato et al. 2015; Miletto Petrazzini et al. 2015b). A third study reported females to be faster than males in discriminating two groups of social companions, but only when the discrimination was difficult (Lucon-Xiccato et al. 2016). The numerical discrimination used here (5 vs. 10) is very easy for guppies; therefore, our results could be attributable to abilities other than a numerical one or to other factors affecting cognitive performance (Griffin et al. 2015; Lucon-Xiccato and Bisazza 2017c).
Regarding sex differences in reversal learning, separate analyses of the two experiments seem to contrast with the results of the previous study in which, despite a similar ability in learning colour discrimination, males made twice as many errors as females in the reversal phase (Lucon-Xiccato and Bisazza 2014). Can we conclude that sex differences in reversal learning are limited to the specific context of colour discrimination? It is worth noting that in both the experiments reported here, females’ average reversal learning performance was better than that of males, with small to medium effect sizes. Bayesian analyses did not reveal convincing evidence in favour of the similarity between the two sexes. Furthermore, our meta-analysis of the three studies on guppies’ reversal learning supports an overall superiority of females. As a consequence, this evidence might suggest that females perform better than males in reversal learning, but this effect is much smaller in the T-maze and numerical discrimination reversal learning than in colour discrimination reversal learning.
The different effect size observed in the three reversal learning experiments can have different explanations. First, despite our sample size being the same as that used in the previous study on guppies’ reversal learning and larger than that of most studies on cognitive sex differences in other non-human animals (e.g. Gaulin and FitzGerald 1986; Liu and Burmeister 2017), it might not be sufficient to ensure effect size stability (Lakens and Evers 2014). However, our simulation based on bootstrapping seems to exclude this possibility: the results observed in this study could not be obtained by randomly resampling the scores of the guppies tested in the reversal learning experiment by Lucon-Xiccato and Bisazza (2014).
A second possibility is that methodological factors have reduced or masked sex differences in the two experiments of this study. We have followed, as far as possible, the same procedure used in the previous study on colour discrimination (Lucon-Xiccato and Bisazza 2014). However, one difference between the experiments is clear: the colour discrimination was learned with few errors by guppies and thus appears much easier than the two discriminations studied here, which were acquired with more than ten errors. So, the degree of training received by subjects may differ between the experiments included in this study and the previous one. Some studies reported that overtraining facilitates reversal learning (rats: Mackintosh 1965), and other studies reported the contrary effect (chicks: Mackintosh 1965; paradise fish: Warren 1960; goldfish: Mackintosh et al. 1966), although it is not clear to which extent this inconsistency of results was due to species rather than procedural differences (Mackintosh et al. 1966). There are no available data on overtraining for guppies or for our specific settings. However, the longer training performed by subjects in the present experiments may have prevented the detection of sex differences in reversal learning. Another methodological issue to consider concerns the strong effect of the rewarded arm’s direction in experiment 1. Sex differences in lateralization are commonly found in vertebrates (Bisazza et al. 1998; Reddon and Hurd 2009; Rilea et al. 2004; Tommasi and Vallortigara 2004). If the turning bias in the T-maze was due to cerebral lateralization, this effect might differ between male and female guppies and might increase performance variance, reducing power to detect learning sex differences.
The third, and perhaps more interesting, explanation for the different effect sizes of the sex difference observed in the present and the previous study regards the presence of sex-specific selective pressures. More flexible responses in colour discrimination learning might have been selected in females, which use colour discrimination for mate choice. Females show an exceptional ability to rapidly estimate and memorize the colour features of the males they encounter, and they use these cues to comparatively evaluate their prospective mates (Houde 1997). They are also extremely flexible in their mate preferences and, for example, prefer males which differ in colour compared to males they were previously exposed to (Eakley and Houde 2004; Hughes et al. 1999). Furthermore, female guppies reverse their mate preference after seeing less preferred males chosen by another female (Dugatkin and Godin 1992; Godin et al. 2005) and modify their colour preferences under perceived predation hazard (Gong and Gibson 1996). Even males have been shown to compare the colouration of potential rivals (Gasparini et al. 2013), but there is no evidence that this ability is highly flexible in this sex. Flexibility in colour discrimination could also undergo sex-specific selective pressures during foraging. Guppies of both sexes have a strong tendency to search for small carotenoid-rich fruits that drop from the forest canopy into the rivers of Trinidad (Rodd et al. 2002). However, for females, fruits enrich the diet with micronutrients that affect physiological functioning, whereas males gain an additional selective advantage because accumulating carotenoids increases their chances of being chosen by females and thus increases male reproductive success (Kodric-Brown 1989). As a consequence, natural and sexual selection could have shaped different foraging strategies and underlying learning mechanisms between the two sexes. For example, it might be that once a source of carotenoids is discovered, it pays for a male to look for similar objects, while ignoring other stimuli. Food imprinting, i.e. foraging bias following previous experience with a specific food type, has been reported in a large number of species (Persons and Rypstra 2000; Burghardt and Hess 1966; Rabinowitch 1969), and there is evidence that the “search image” (Tinbergen 1960) can be based on colour (Croze 1970). Conversely, for females, who are called on to maximize embryo production, it might be more important to exploit caloric intake, opportunistically utilizing every source of food, and this could have promoted the switch from one type of food to another (Laland and Reader 1999). More research is needed to disentangle the possible explanations for task-specific sex differences in reversal learning.
In conclusion, despite the absence of clear sex differences in the two reversal learning experiments presented here, this study does not overtly exclude the hypothesis that female guppies are generally more flexible than males in reversal learning as observed in other vertebrates. Notably, our study suggests that guppies’ sex differences in reversal learning might vary across cognitive tasks. This points out the importance of investigating sex differences in fish cognitive flexibility with different reversal learning tasks controlling for methodological factors that can affect flexibility; also, it would be important to use other types of tests to measure cognitive flexibility.
References
Agrillo C, Bisazza A (2014) Spontaneous versus trained numerical abilities. A comparison between the two main tools to study numerical competence in non-human animals. J Neurosci Met 234:82–91
Agrillo C, Miletto Petrazzini ME, Bisazza A (2017) Numerical abilities in fish: a methodological review. Behav Process 141:161–171
Barchard KA (2015) Null hypothesis significance testing does not show equivalence. Anal Soc Issues Public Policy 15:418–421
Bisazza A, Brown C (2011) Lateralization of cognitive functions in fish. In: Brown C, Krause J, Laland KN (eds) Fish cognition and behavior. Wiley, Oxford, pp 298–324
Bisazza A, Facchin L, Pignatti R, Vallortigara G (1998) Lateralization of detour behaviour in poeciliid fish: the effect of species, gender and sexual motivation. Behav Brain Res 91:157–164
Brust V, Wuerz Y, Krüger O (2013) Behavioural flexibility and personality in zebra finches. Ethology 119:559–569
Burghardt GM, Hess EH (1966) Food imprinting in the snapping turtle, Chelydra serpentina. Science 151:108–109
Crawley MJ (2012) The R book. Wiley, Chichester
Croze H (1970) Searching image in carrion crows. Paul Parey, Berlin
Dugatkin LA, Godin JGJ (1992) Reversal of female mate choice by copying in the guppy (Poecilia reticulata). Proc R Soc Lond Ser B Biol Sci 249:179–184
Eakley AL, Houde AE (2004) Possible role of female discrimination against ‘redundant’ males in the evolution of colour pattern polymorphism in guppies. Proc R Soc Lond Ser B Biol Sci 271:S299–S301
Elias MF, Dupree M, Eleftheriou BE (1973) Differences in spatial discrimination reversal learning between two inbred mouse strains following specific amygdaloid lesions. J Comp Physiol Psychol 83:149–156
Feigenson L, Carey S, Spelke E (2002) Infants’ discrimination of number vs. continuous extent. Cogn Psychol 44:33–66
Gasparini C, Serena G, Pilastro A (2013) Do unattractive friends make you look better? Context-dependent male mating preferences in the guppy. Proc R Soc Lond Ser B Biol Sci 280:20123072
Gaulin SJ, FitzGerald RW (1986) Sex differences in spatial ability: an evolutionary hypothesis and test. Am Nat 127:74–88
Gebuis T, Reynvoet B (2012) The interplay between nonsymbolic number and its continuous visual properties. J Exp Psychol Gen 141:642–648
Godin JGJ, Herdman EJ, Dugatkin LA (2005) Social influences on female mate choice in the guppy, Poecilia reticulata: generalized and repeatable trait-copying behaviour. Anim Behav 69:999–1005
Gómez-Laplaza LM, Gerlai R (2010) Latent learning in zebrafish (Danio rerio). Behav Brain Res 208:509–515
Gong A, Gibson RM (1996) Reversal of a female preference after visual exposure to a predator in the guppy, Poecilia reticulata. Anim Behav 52:1007–1015
Grether GF (2000) Carotenoid limitation and mate preference evolution: a test of the indicator hypothesis in guppies (Poecilia reticulata). Evolution 54:1712–1724
Griffin AS, Guillette LM, Healy SD (2015) Cognition and personality: an analysis of an emerging field. Trends Ecol Evol 30:207–214
Guillamón A, Valencia A, Calés J, Segovia S (1986) Effects of early postnatal gonadal steroids on the successive conditional discrimination reversal learning in the rat. Physiol Behav 38:845–849
Ha JC, Mandell DJ, Gray J (2011) Two-item discrimination and Hamilton search learning in infant pigtailed macaque monkeys. Behav Process 86:1–6
Houde AE (1997) Sex, color, and mate choice in guppies. Princeton University Press, Princeton
Hughes KA, Du L, Rodd FH, Reznick DN (1999) Familiarity leads to female mate preference for novel males in the guppy, Poecilia reticulata. Anim Behav 58:907–916
Jeffreys H (1961) Theory of probability, 3rd edn. Oxford University Press, Oxford
Kellogg WN, Gavin J (1960) Maze-learning in the guppy. Psychol Rep 6:445–446
Kodric-Brown A (1989) Dietary carotenoids and male mating success in the guppy: an environmental component to female choice. Behav Ecol Sociobiol 25:393–401
Lakens D, Evers ER (2014) Sailing from the seas of chaos into the corridor of stability: practical recommendations to increase the informational value of studies. Perspect Psychol Sci 9:278–292
Laland KN, Reader SM (1999) Foraging innovation in the guppy. Anim Behav 57:331–340
Liu Y, Burmeister SS (2017) Sex differences during place learning in the túngara frog. Anim Behav 128:61–67
Lucon-Xiccato T, Bisazza A (2014) Discrimination reversal learning reveals greater female behavioural flexibility in guppies. Biol Lett 10:20140206
Lucon-Xiccato T, Bisazza A (2016) Male and female guppies differ in speed but not in accuracy in visual discrimination learning. Anim Cogn 19:733–744
Lucon-Xiccato T, Bisazza A (2017a) Sex differences in spatial abilities and cognitive flexibility in the guppy. Anim Behav 123:53–60
Lucon-Xiccato T, Bisazza A (2017b) Complex maze learning by fish. Anim Behav 125:69–75
Lucon-Xiccato T, Bisazza A (2017c) Individual differences in cognition among teleost fishes. Behav Process 141:184–195
Lucon-Xiccato T, Miletto Petrazzini ME, Agrillo C, Bisazza A (2015) Guppies discriminate between two quantities of food items but prioritize item size over total amount. Anim Behav 107:183–191
Lucon-Xiccato T, Dadda M, Bisazza A (2016) Sex differences in discrimination of shoal size in the guppy (Poecilia reticulata). Ethology 122:481–491
Mackintosh NJ (1965) Overtraining, reversal, and extinction in rats and chicks. J Comp Physiol Psychol 59:31–36
Mackintosh NJ, Mackintosh J, Safriel-Jorne O, Sutherland NS (1966) Overtraining, reversal and extinction in the goldfish. Anim Behav 14:314–318
Mandler G, Shebo BJ (1982) Subitizing: an analysis of its component processes. J Exp Psychol Gen 111:1–22
Miletto Petrazzini ME, Agrillo C, Izard V, Bisazza A (2015a) Relative versus absolute numerical representation in fish: can guppies represent “fourness”? Anim Cogn 18:1007–1017
Miletto Petrazzini ME, Lucon-Xiccato T, Agrillo C, Bisazza A (2015b) Use of ordinal information by fish. Sci Rep 5:15497
Persons MH, Rypstra AL (2000) Preference for chemical cues associated with recent prey in the wolf spider Hogna helluo (Araneae: Lycosidae). Ethology 106:27–35
Pisa PE, Agrillo C (2009) Quantity discrimination in felines: a preliminary investigation of the domestic cat (Felis silvestris catus). J Ethol 27:289–293
Rabinowitch V (1969) The role of experience in the development and retention of seed preferences in zebra finches. Behaviour 33:222–235
Reddon AR, Hurd PL (2009) Sex differences in the cerebral lateralization of a cichlid fish when detouring to view emotionally conditioned stimuli. Behav Process 82:25–29
Rilea SL, Roskos-Ewoldsen B, Boles D (2004) Sex differences in spatial ability: a lateralization of function approach. Brain Cogn 56:332–343
Rodd FH, Hughes KA, Grether GF, Baril CT (2002) A possible non-sexual origin of mate preference: are male guppies mimicking fruit? Proc R Soc Lond Ser B Biol Sci 269:475–481
Roelofs S, Nordquist RE, van der Staay FJ (2017) Female and male pigs’ performance in a spatial holeboard and judgment bias task. Appl Anim Behav Sci. doi:10.1016/j.applanim.2017.01.016
Rogers LJ (1974) Persistence and search influenced by natural levels of androgens in young and adult chickens. Physiol Behav 12:197–204
Rowe L, Cameron E, Day T (2005) Escalation, retreat, and female indifference as alternative outcomes of sexually antagonistic coevolution. Am Nat 165:S5–S18
Shettleworth SJ (2010) Cognition, evolution, and behavior. Oxford University Press, Oxford
Tinbergen L (1960) The natural control of insects in pinewoods. Arch Neerl Zool 13:265–343
Titulaer M, van Oers K, Naguib M (2012) Personality affects learning performance in difficult tasks in a sex-dependent way. Anim Behav 83:723–730
Tommasi L, Vallortigara G (2004) Hemispheric processing of landmark and geometric information in male and female domestic chicks (Gallus gallus). Behav Brain Res 155:85–96
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw 36:1–48
Wagenmakers EJ (2007) A practical solution to the pervasive problems of p values. Psychon Bull Rev 14:779–804
Warren JM (1960) Reversal learning by paradise fish (Macropodus opercularis). J Comp Physiol Psychol 53:376–378
Watson DJ, Stanton ME (2009) Intrahippocampal administration of an NMDA-receptor antagonist impairs spatial discrimination reversal learning in weanling rats. Neurobiol Learn Mem 92:89–98
Acknowledgements
We would like to thank Immacolata Orrù and Jacqueline Giada Mazzon for their help in testing the animals. This study was founded by “PRIN 2015” (prot.: 2015FFATB7) from the University of Padova to Angelo Bisazza.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
Experiments were conducted in agreement with the law of our country (Italy, D.L. 4 Marzo 2014, n. 26). The Ethical Committee of the University of Padova reviewed and approved the experimental procedures (Protocol n. 33/2015 and n. 22/2016). None of the subjects showed sign of distress. At the end of the experiments, all subjects were released into the maintenance tanks.
Rights and permissions
About this article
Cite this article
Miletto Petrazzini, M.E., Bisazza, A., Agrillo, C. et al. Sex differences in discrimination reversal learning in the guppy. Anim Cogn 20, 1081–1091 (2017). https://doi.org/10.1007/s10071-017-1124-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-017-1124-4