Abstract
We evaluated radio frequency (RF) dielectric heating for eradication of pinewood nematodes (PWN) in infested wood. Thirteen temperatures were tested (from ambient to 70 °C) on small wood samples (2.5 × 3.8 × 0.64 cm) to determine the minimum lethal temperature (100 % mortality), which was 56 °C [based on infra-red (IR) thermal images data 55.5–57.4 °C] with a 1 min hold time. We also used thermal probes inside the wood to confirm that temperatures were ≥56 °C. Thirty additional samples were tested bracketing the minimum lethal temperature using 54, 56 and 58 °C with additional replications to produce the minimum sample size equivalent of 100 % mortality of at least 93,616 nematodes to satisfy the Probit 9 efficacy requirement. This minimum lethal temperature was further verified by treating infested large wood blocks (10.2 × 10.2 × 25.4 cm). All samples that met or exceeded the 56 °C lethal temperature for the required 1 min hold time (as measured by probes inserted in the wood and on the wood surface by IR) produced 100 % mortality. The sample size required to show Probit 9 efficacy was also satisfied. This study supports the consideration of RF in addition to microwave (MW) dielectric heating as alternative treatments of wood packaging material for inclusion in ISPM No. 15, provided the treatment delivers the target lethal temperature throughout the profile of the material in industrial scale operations.
Similar content being viewed by others
Introduction
It has been well documented that numerous plant pests and pathogens are moved around the world via international trade of various commodities [1]. Some of these introductions have caused significant economic and ecological losses, and with increasing trading volume, there is a concomitant increase in introductions of alien species. Since wood packaging was recognized as one of the most important pathways for introductions of forest-related pests, the International Plant Protection Convention (IPPC), which is an international agreement among (currently) 177 countries, adopted the International Standard for Phytosanitary Measures No. 15 (ISPM No 15) in 2002; this measure serves as a guideline for the regulation of wood packaging material used in international trade [2]. Only two phytosanitary treatments are currently approved under ISPM No. 15: conventional heat treatment (HT) and methyl bromide fumigation. HT is usually done using kilns with the requirement for the wood to reach a minimum of 56 °C held for 30 min at the core of the wood. While conventional HT has been shown to be an effective method to sanitize wood from invasive pests, it may be energy intensive, time consuming and sometimes not feasible. The second method is methyl bromide fumigation under specific protocols. In an effort to support the initiatives to phase out the use of methyl bromide in compliance with the Montreal Protocol, the use of which is already restricted in some countries, and to provide the industry with alternative treatment methods, new approaches have been investigated and submissions of data supporting these new treatments are encouraged.
A potential alternative for phytosanitary treatment of wood packaging material is dielectric heating by radiofrequency (RF) or microwaves (MW). Dielectric heating is an effective technique used to sanitize other commodities such as food, with significantly reduced treatment times compared to conventional heat [3, 4]. For wood materials, heating time to reach target temperatures is typically achieved in minutes, while conventional heat typically requires hours [5, 6]. Although definitive estimations of energy consumption and overall economics are not currently available and beyond the scope of the current study, the disparity in treatment times is great enough to explore the efficacy of dielectric heating for phytosanitation of wood packaging.
Several studies have shown the effectiveness of using dielectric heating to sanitize wood from invasive pests, particularly pinewood nematode (PWN; Bursaphelenchus xylophilus). Because PWN is a primary casual agent of pine wilt and considered to be a major threat to conifer forests, especially pines in Asia and Portugal, it is one of the key pests required to be tested in efficacy trials for new treatments [2]. Research by Hoover et al. [7] revealed that microwave heating effectively eradicates (100 % efficacy at Probit 9 level) PWN in infested, laboratory scale wood samples (2.5 × 3.8 × 0.64 cm) and commercial sized samples (8.9 × 8.9 × 25.4 cm) when 56 °C is maintained throughout the profile of the sample for 1 min. Exploratory work to evaluate RF/vacuum heating for eradicating PWN was reported by Dwinell et al. [8]. The greatest efficacy was achieved when wood was heated to a temperature greater than 56 °C prior to drying and the authors recognized the potential of RF as a rapid pasteurization treatment. They also noted, however, high variability in temperatures within the heated boards. Successful use of RF to eradicate PWN has been shown for wood with a cross section under 4.0 × 9.0 cm [9]. In this case, the authors used RF heating to achieve the ISPM-15 heat treatment requirement (56 °C/30 min) and they have shown that boards heated following this protocol fully eradicated PWN. The authors also showed that 100 % mortality could be obtained at 60 °C held for 15 min. However, additional work is necessary to confirm that, similar to MW [7], RF would be as effective in killing PWN using shorter treatment times.
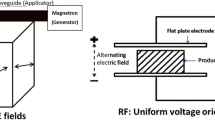
The use of RF ovens for dielectric heating of wood materials is similar to MW technology, but differences exist in how the high frequency electric field is applied. MW technology employs a magnetron to produce the radiation that is bombarded onto the surface of the wood. In RF ovens, the wood material absorbs the radiation of an RF electric field that is created between two electrodes. Industrial RF heating employs an electric field at a frequency much lower (13.56 and 27.12 MHz) compared to MW (915 and 2,450 MHz). The lower frequency (larger wavelength) leads to a disparity in depth of penetration since it is proportional to wavelength [10]. The following formula (Eq. 1) gives characteristic depth of penetration as a function of the wavelength for effective dielectric heating with respect to critical material parameters:
where D p is the depth of penetration, \( \lambda^{\prime}_{0} \) the wavelength, \( \in^{\prime} \) the dielectric constant of the material being heated, and \( \in^{\prime\prime}_{\text{eff}} \) is the effective loss factor of material being heated.
This formula clearly shows that RF has a greater depth of penetration than MW because the wavelength for RF is longer, such that it can treat thicker wood materials (dunnage) or be used for bulk volume treatment of stacked packaging material. This is an important advantage of RF over MW. However, until the work reported here was performed, it had not been ascertained if the difference in frequency and electric field would have a negative influence on the mortality of PWN using markedly reduced treatment duration (1 min compared to 30 min hold time). Furthermore, for the shorter treatment duration, heating variability could be more prevalent and the likelihood of improper treatment increased. Thus, it was imperative to determine if the methodology and treatment schedule developed by Hoover et al. [7] using MW would produce sufficient efficacy against PWN using RF.
Materials and methods
Experiments were conducted in two steps following the experimental design outlined by Hoover et al. [7]. The objective of Step 1 was to determine the minimum lethal temperature using RF energy required to kill 100 % of PWN with a short hold time. Although the lethal temperature with a 1 min hold time had been determined previously for MW [7], because the frequency and thus depth of penetration using RF differs from MW, there was no guarantee that these parameters would be the same. The objective of Step 2 was to verify the lethal temperature in commercial sized wood samples.
For all experiments, we used a PSC manufactured oven system, which is a 15 kW dielectric heating capacity research designed unit (Model No: PP15L, Serial No: 894 built by PSC for USDA, APHIS, Worchester, MA). It is an oscillator-based oven that delivers continuous RF power output with an operational frequency of 19.0 MHz. Electrode spacing can be independently adjusted from 10.2 to 25.6 cm from the load being treated. Plate height was adjusted to achieve optimum operating conditions as recommended by the manufacturer. Plate voltage (KV) was 10.5 KVDC and grid current was 0.35 amp. Plate current reaches 2.5 amp when the oven is fully loaded. Prior to experimentation the RF oven was calibrated for optimum heating consistency.
Step 1: Determination of minimal lethal temperature for PWN-infested wood using RF
Sample preparation was performed as described previously for MW treatment [7]. In short, fresh lodgepole pine (Pinus contorta var. latifolia Engelm) sapwood was cut into 2.5 × 3.8 × 0.64 cm samples and sterilized with 25 kiloGrays of ionizing irradiation. Samples were then inoculated with a hyphae/spore mixture of the blue stain fungi Leptographium terebrantis, L. longiclavatum, Ophiostoma montium and O. clavigerum, the wood decay fungi Phellinus chrysoloma and Trichaptum abietinum, and Botrytis cinerea. Samples were incubated at 25 °C for 14 days to allow fungi to colonize the wood. Each sample was then inoculated with 1,500 nematodes in 150 μl sterile water and incubated for several weeks prior to treatment to allow nematodes to multiply and establish throughout the sample. Prior to treatment, nematode populations of 12 randomly chosen wood samples were assessed using the Baermann funnel method [11, 12] to insure adequate colonization of samples. This is done by splitting the wood samples into matchstick size pieces and submersing them in water in the Baermann funnel for 24 h, after which nematode populations were counted to determine the mean number of nematodes per gram of wood.
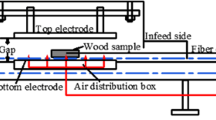
The microscopic size and random distribution of PWN do not allow the measurement of direct body temperature of the nematode, thus the temperature of the wood material is used as a secondary measure to determine the lethal temperature. Consequently, any differences in the heating process that alter how the wood is heated could influence efficacy. For RF treatment, the wood samples were enclosed in an encapsulating wood cube (8.9 × 8.9 × 8.9 cm) as described previously [7] so that the specimens would retain heat for the desired treatment time. The only difference was that wood cubes were constructed from lodgepole pine instead of northern red pine (Pinus resinosa).
To estimate the lethal temperature, 10 small wood samples infested with nematodes were treated with RF irradiation at 13 temperatures: 40, 48, 50, 52, 54, 55, 56, 58, 60, 63, 65, 67 and 70 °C. Fifteen untreated control samples were handled in the same way as the treated samples but only subjected to ambient temperature (~20 °C). For treatment, the encapsulating blocks containing the test sample were placed with the longitudinal grain orientation perpendicular to the electrodes and the sample oriented in a flat manner parallel to the electrodes (see Fig. 1a). The oven was adjusted as recommended by the manufacturer, at an electrode height of 22.9 cm and 100 % KV. During treatment, internal sample temperatures were continuously monitored with three fiber optic probes (Neoptix, Quebec City, Canada) originally positioned as described previously [7] (see Fig. 1b). The RF oven was shut off when the last of the three temperature probes reached the target temperature and the samples were left inside the oven for a 1 min hold time. Following the hold time, the encapsulating blocks were immediately opened and the adjoining inner surfaces of the block containing the test sample were photographed with a FLIR Thermacam E65 camera (FLIR, Wilsonville, OR) to produce infrared images of the sample. The samples were then removed from the encapsulating block and weighed (both before and after treatment) to monitor water loss due to RF heating, and then individually wrapped in plastic bags until assessment of PWN survival. Each encapsulating block was submerged in water for 6–10 min after treatment to insure the moisture content of the encapsulating blocks remained consistent after each use, thus limiting variability in heating with respect to the moisture gradient between the sample and block.
Following the temperature screening described above, we improved heating consistency using a configuration setup with the sample sandwiched between two polyethylene plates and two aluminum plates (Fig. 2a) with an electrode height of 27.9 cm and 100 % KV [7]. We also changed the probe configuration as shown in Fig. 2b to better capture possible variation of temperature within the test wood. With this new setting, we completed Step 1 by treating 30 replicates containing enough nematodes to meet the Probit 9 mortality standard at the lethal temperature and at temperatures above and below 2 °C, i.e., bracketing the lethal temperature. The population density in test wood samples was used to determine the required number of replicates necessary to achieve efficacy at Probit 9. Probit 9 historically represents killing 99.9968 % of the target organisms in a sample of ≥100,000 organisms, or 100 % mortality of a minimum of 93,613 individuals [13, 14].
Post-treatment assessment of nematode populations
Following the RF treatment described above, the individually wrapped samples were incubated for 6 days and were split into three parts (Fig. 3a). Sample A was used for gravimetric moisture content (MC) measurement [15], sample B was tested for nematode survival (6-day assessment) using the Baermann funnel method [11, 12], and sample C was incubated for 21 days and tested for nematode survival if the 6-day assessment (sample B) showed no survivors. Nematode populations in the control samples were also counted after 6 days of incubation. Percentage mortality was calculated for each treatment temperature based on the minimum temperature achieved, as recorded by the fiber optic probes.
Step 2: Verification of lethal temperature for PWN infested, commercial sized wood samples
For Step 2, PWN-infested logs were prepared as described previously [7]. In short, freshly cut P. contorta logs were inoculated with the same mix of fungi as used in Step 1. Logs were incubated and kept moist under lumber wrap for approximately 3 weeks. Logs were then inoculated with PWN and incubated under the same conditions to allow nematodes to multiply and spread throughout the sapwood. Commercial sized blocks (10.2 × 10.2 × 25.4 cm) were cut from logs leaving 5.0 cm sections in between each sample block as control samples for estimating the number of nematodes per gram of wood, moisture content, and sapwood/heartwood ratio (Fig. 4).
Three treatment configurations were tested to determine optimal time to reach target temperature, lowest level of temperature overshoot, and evenness of heating throughout the sample as follows: (1) a polyethylene plate was placed underneath the sample, (2) a polyethylene/aluminum plate was placed on both the top and bottom of the sample, and (3) the sample was fully encased in a 4 cm thick ponderosa pine box using only the polyethylene plate underneath. Due to the extent of evaporative cooling on the surface of the large samples in the first two configurations, the third configuration using the encasing wood box (Fig. 5a) was chosen because it resulted in the most even heating pattern from inner to outer surface temperatures (data not shown).
Ten replicates of the commercial sized blocks were treated at 58 and 60 °C, and two were used for controls. Based on the estimated average number of nematodes per gram of sapwood, the 10 blocks provided the required number of nematodes to meet the Probit 9 requirement of over 93,613 individual nematodes. Internal temperatures were monitored at eight different locations in the sample block using fiber optic temperature probes. The guide holes for the probes were drilled in strategic positions to try to capture representative temperatures of the entire block (Table 1; Fig. 5b). Immediately after the last temperature probe had reached the target temperature and following the 1 min hold time, the sample was removed from the oven, taken out of the encapsulating block, placed in front of the IR camera, and all six surfaces were imaged. The sample was then crosscut in two positions: the first position was located 2.5 cm from the front of the sample and the second position was located at the approximate center of the block. These two inner faces were then imaged. The three pieces were then realigned, placed in a plastic bag, incubated for 6 days at room temperature, and then assessed for nematode survival.
Pre- and post-assessment of nematode populations in commercial sized wood samples
For pre-assessments of nematode populations, the control sections were processed on the day of or the day after the large sample block was treated. The proportion of sapwood (on a wet weight basis) was calculated as described previously [7] and the nematode population expressed as the number of nematodes per gram of sapwood. Four 5–10 g of wood were removed from the sapwood (Fig. 3b), chiseled into small pieces, weighed, and processed using the Baermann funnel method. The mean number of nematodes per gram of sapwood (green) was calculated for each sample block. In addition, two pieces of wood were chiseled from the control section for MC determination. The heartwood was also dried and the MC calculated. The total weight of each sample was converted to dry weight, and the mean number of nematodes per gram of oven dried wood was also calculated.
Post-treatment nematode assessments were conducted at 6 and 21 days. The treated blocks were kept in a plastic box covered in plastic wrap to retain moisture until they were assessed. Five 3–6 g wood samples from random locations on the sapwood sides of the treated blocks were collected using a hand drill fitted with a 20 mm diameter auger wood boring bit. The samples were processed in Baermann funnels and nematode populations counted after 24–48 h. Nematode numbers from day 6 assessments of control samples were counted to insure that at least 93,613 nematodes were treated at the minimum temperature that produced 100 % mortality.
Results
Step 1: Screening for lethal temperature using replication of 10 blocks per temperature
Pre-assessment of nematode populations resulted in an average of 5,409 ± 867.8 (SE) nematodes (range 1,210–12,025 nematodes) per test block, resulting in an average of 54,090 nematodes tested at each temperature.
A large amount of variability in the temperature profiles was observed with RF heating. When the oven was turned off after the last probe had reached target temperature, and after the 1 min hold time, the probes showed temperatures ranging from 10 °C below target temperature to up to 48 °C above target temperature (an overshoot). Although all three probes had reached the target temperature, the IR images of some of the samples showed cold areas within the sample with temperatures as much as 20 °C below target (Fig. 6). Table 2 shows survival data based on temperature probes; there was nematode survival in at least one of the ten samples for all temperatures screened (except for 67 °C).
IR image of a thoroughly heated sample that had no nematode survival (a) and of a sample that had nematode survival due to a cold spot (b). Target temperature for both was 56 °C. Note that the temperature range is exclusively for the boxed area that covers the surface of the inoculated sample (cold spot is blue in color) (color figure online)
Since the minimum temperature from each IR image was recorded and compared with the survival data from the corresponding sample, we were able to calculate percent mortality for each temperature range (Table 3). Based on these data, the minimum temperature range that resulted in 100 % mortality was 55.5–57.4 °C.
Step 1: Bracketing of minimum lethal temperature with higher replication
Since the minimum lethal temperature we observed was 56 °C (rounded up from 55.5 °C), 30 replicates were treated at this temperature and at 2 °C below and above the lethal temperature, i.e., 54 and 58 °C to satisfy the Probit 9 mortality requirement. In our experiments, nematode survival was observed in 13, 3, and 2 replicates of each of these temperatures, respectively (Table 4). However, all samples with nematode survival displayed temperatures below 56 °C by the end of the 1 min hold time, according to the fiber optic probe data and/or the IR images (Fig. 7). Also, all samples that resulted in 100 % dead nematodes maintained temperatures above 56 °C for the duration of the 1 min hold time. The moisture loss per sample following RF treatment ranged from 2 to 10 %.
IR images of both failed (top row) and successfully treated (bottom row) samples at each temperature photographed immediately after the halves of encapsulating blocks were opened (only the halves containing the test samples were photographed, boxed region on the image contained the inoculated specimen) (color figure online)
Based on the nematode counts from the control samples, an average of 98,070 nematodes were treated at each temperature. Coupled with the treated nematodes from the initial screening experiment described in the above section, well over 100,000 nematodes were treated at the minimum lethal temperature, satisfying the Probit 9 requirement.
Step 2: Verification of minimum lethal temperature using industrial scale, large wood blocks
The average moisture content of sapwood and heartwood (N = 30 blocks) was 97.7 ± 3.1 (SE) % and 35.0 ± 0.3 (SE) %, respectively, in the large wood blocks. The average pre-treatment wet weight of the sample blocks was 1,318 ± 15.5 g/block, with mean sapwood weight of 713.5 ± 27.4 (SE) g/block. The calculated heartwood portion was 604.5 g, thus the ratio of sapwood to heartwood was 1.18. Pre-treatment nematode populations averaged 110 ± 19.4 (SE) nematodes per gram of sapwood dry weight in treated blocks. Thus, for the two treatment temperatures, 58 and 60 °C, a total of 432,462 and 834,550 nematodes were treated, respectively.
The use of the encapsulating box for the large wood samples drastically reduced evaporative cooling from the surface of the samples, which resulted in a more consistent heating profile throughout the sample surface. Due to the variability in heating and the occurrence of cold spots observed in Step 1, 58 °C was chosen as the minimum target temperature for Step 2 along with a second temperature of 60 °C. During the preliminary configuration experiments, five blocks were treated at 60 °C inside the encapsulating block and therefore, total sample sizes for Step 2 were 10 at 58 °C and 15 at 60 °C. No survival was found in any of the 15 treatments at 60 °C. Nematode survival was observed in only one block treated at 58 °C (Table 5). It was observed for the replicate with nematode survival that one of the eight probes measured 55.7 °C before the end of the 1 min hold time. IR images taken from all six surfaces around the block and from the two inner faces after the sample was cut at two positions also showed temperatures lower than 56 °C (Fig. 8). Table 5 presents the number of live nematodes in the sample that failed to maintain target temperature through the profile for 1 min. For that failed sample, nematodes were found in 3 of the 5 sapwood samples processed after 6 days of incubation. After 21 days of incubation, live nematodes were found in all sapwood samples examined and their number increased.
Discussion
Overall, all samples in which 100 % of PWN were killed maintained internal and surface temperatures of ≥56 °C for 1 min, according to the fiber optic probe data and/or the IR images. Samples for which survival occurred failed to maintain the minimum lethal temperature through the profile of the wood. These results indicate that the minimum temperature required to produce greater than 99.99638 % mortality (Probit 9) using RF energy was ≥56 °C held for 1 min both in small wood samples (Step 1) and in commercial sized wood (Step 2). This result confirms that the lethal temperature for PWN using dielectric heating, whether it is performed with RF or MW [7], is the same. This result suggests that the efficacy of dielectric heating is not related to frequency or the arrangement of the dielectric field, but the maintenance of a lethal temperature for a specified duration. Therefore, RF and MW may be considered equivalent forms of dielectric heating for the purpose of phytosanitation, provided the lethal temperature is achieved and maintained through the profile of the material for at least 1 min.
Although the results showed that mortality occurred at the same lethal temperature, RF treatment yielded less consistent heating patterns compared to MW. Cold spots occurred in some samples despite the fact that all three (Step 1) or eight fiber optic probes (Step 2) reached the targeted temperature. The variability of heating patterns resulted in survival of at least one of the ten samples for all temperatures screened (except for 67 °C) in small blocks, and to a lesser extent in large wood blocks. The failed samples (in Step 1) at 56 and 58 °C had cold spots according to their IR images (Fig. 7).
In Step 2, nematode survival was observed in only one block treated at 58 °C and again we found that one of the eight probes had dropped in temperature to 55.7 °C during the 1 min hold time, and IR images taken from eight surfaces of the same sample showed temperatures consistently lower than 56 °C. These results highlight the importance of reaching and holding the minimal lethal temperature throughout the entire sample. Any occurrence of cold spots or areas that do not reach the lethal temperature may result in pest survival. The survival of even a small number of nematodes may lead to repopulation of the sample, as seen in the increase in nematode population from the day 6 to 21 incubation period in the failed sample.
Inconsistency in heating could have resulted from the relatively small load relative to the size of the RF oven. RF ovens are less efficient in heating small units of material (such as our test material) since the dielectric field can become distorted over a small material or due to a geometry effect where there is a limited amount of treating surface in relation to the size of the oven electrode plates. Heat distribution using RF will also be influenced by the amount of energy used, design and type of oven, type of load (batch, single, continuous), and characteristics of the wood being heated (anatomy, moisture content, etc.). In this study, a straightforward approach was taken to improve heating consistency using aluminum plates that concentrate energy, creating virtual electrodes that are closer to the treated wood (Lazaresku Ciprian, personal communication), which increases speed of heating. The polyethylene sandwich also concentrated energy by deflecting the field into the sample. In an industrial setting, a full load would occupy the space between electrodes and, therefore, represent a similar situation. Because a range of parameters exist that can affect the temperature distribution in the wood material, it is currently difficult to determine a set of optimal operating parameters that would maximize heating consistency in all situations.
Despite the lack of optimum operating conditions, this study revealed that the negative effects of heating inconsistency were mitigated through meticulous monitoring of temperature. The methodology developed by Hoover et al. [7] proved to be sufficient in identifying inadequate treatment and explaining cases of nematode survival, provided fiber optic probes are located in optimal positions to locate cold spots. The probes were used for primary temperature observation and control because of their reliability, but this means relying on the measured temperatures of finite locations within the wood, which do not represent all points in the wood. After preliminary trials in Step 1, it was observed that cold spots appeared in similar locations, so the probes were repositioned accordingly. Although the probe relocations reduced the risk of cold spots, a secondary macroscopic technique was required to determine the existence of cold spots across the entire specimen. When we used IR images to measure the surface temperature of treated wood, we could detect cold spots, which explained survival of nematodes despite the fact that all of the internal fiber optic probes reached or exceeded the target temperature.
Rather than using operational parameters to guide the treatment process, effective treatment can be assessed exclusively through temperature control. The key question will be how to monitor temperature in a commercial treatment setting to insure that the target temperatures are delivered throughout the profile of the wood for the duration of the hold time. It is very likely that, in the industrial process, especially continuous processes, IR cameras will be used for reading surface temperature. Hoover et al. [7] showed that surface temperature represented the coldest region of the heated specimen during MW treatment because of an evaporative cooling effect. Consequently, monitoring surface temperatures to insure that lethal temperatures are achieved through the profile of the wood was a practical solution during MW heating. Figures 6, 7, and 8 support these findings, as the surface is generally cooler than the interior of the specimen, and at least one surface is as cold as the coldest spot of the interior.
With further development, IR technology has the potential to be used as a temperature control method for dielectric heating of wood packaging material. Under commercial conditions, wood that did not hold the lethal temperature for at least 1 min would be re-treated to prevent treatment failure. Moreover, a safety factor can be implemented to alleviate the concern of under-treatment, which would require maintaining temperatures several degrees above the lethal temperature of 56 °C. Interestingly in earlier experimental work that was instrumental in developing conventional heat treatment schedules against PWN, it was found (using a heated water bath) that 100 % of PWN were killed at 52 °C with 30 min hold time, while 56.1 °C was selected from a mortality curve (fitted Gompertz dose response model) following these experiments to give 99.9994 probability of 100 % mortality [16]. The 56/30 (achieving 56 °C to the core of the wood for a 30 min hold time) has proved successful in practice under commercial conditions to date.
In order for dielectric technologies to become commercially viable, collaboration with industry is necessary to develop methodologies for proper temperature monitoring at larger scales. MW and RF have competing advantages and disadvantages. While MW currently appears to heat more consistently, it does not penetrate wood as deeply, which could limit productivity. Temperature distribution in wood using RF is less consistent, but could be the more viable option in the future because of its ability to penetrate deeper into the wood, such that a larger amount of material may be treated simultaneously. In addition, bulk treating with a slower rise time to reach target temperature may prove to solve the problem of unequal heating. These types of studies are underway by our group.
Conclusions
The present study showed that dielectric heating using RF can be used to successfully sanitize wood material infested with PWN on a lab scale, providing that the minimum lethal temperature of 56 °C is achieved and held for 1 min through the profile of the wood. Further development is necessary to insure that the lethal temperature can be reached and maintained through the profile of the wood on an industrial scale.
References
Leal I, Allen E, Humble L, Sela S, Uzunovic A (2010) Phytosanitary risks associated with the global movement of forest products: A commodity-based approach. Natural Resources Canada, Canadian Forest Service, Pacific Forestry Centre, Victoria, BC. Information Report BC-X-419. http://publications.gc.ca/collections/collection_2011/rncan-nrcan/Fo143-2-419-eng.pdf. Accessed 23 May 2012
Food and Agriculture Organization (FAO) (2009a) International standards for phytosanitary measures: guidelines for regulating wood packaging material in international trade. ISPM No. 15. Rome, Italy
Wang S, Tang J (2001) Radio frequency and microwave alternative treatments for insect control in nuts. Agric Eng J 10(3&4):105–120
Wang Y, Wig TD, Tang J, Hallberg LM (2003) Sterilization of foodstuffs using radio frequency heating. J Food Sci 68(2):539–544
Watanabe K, Abubakari A, Lazarescu C, Avramidis S (2011) Softwood heating in radio frequency fields. Eur J Wood Prod 69:295–301
Zielonka P, Gierlik E (1999) Temperature distribution during conventional and microwave heating. Holz als Roh- und Werkstoff 57:247–249
Hoover K, Uzunovic A, Gething B, Dale A, Leung K, Ostiguy N, Janowiak JJ (2010) Lethal temperature for pinewood nematode, Bursaphelenchus xylophilus, in infested wood using microwave energy. J Nematol 42(2):101–110
Dwinell LD, Avramidis A, Clark JE (1994) Evaluation of radio-frequency/vacuum dryer for eradicating the pinewood nematode in green sawn wood. For Prod J 44(4):19–24
Lazarescu C, Dale A, Uzunovic A, Breuil C, Avramidis S (2011) Radio frequency heating pasteurization of pine wood nematode (Bursaphelenchus xylophilus) infected wood. Eur J Wood Wood Prod 69:573–578
Metaxas AC, Mereith RJ (1983) Industrial microwave heating. Peter Peregrinus Ltd., London, p 80
Baermann G (1917) Eine einfache Methode zur Auffindung von Ankylostomum (nematoden) Larven in Erdproben. Geneesk Tijdschr Ned-Indië 57:131–137
Southey JF (ed) (1986) Laboratory methods for work with plant and soil nematodes. Ministry of Agriculture, Fisheries and Food, Reference Book 402, London
Schortemeyer M, Thomas K, Haack R, Uzunovic A, Hoover K, Simpson JA, Grgurinovic CA (2011) Appropriateness of Probit-9 in the development of quarantine treatments for timber and timber commodities. J Econ Entomol 104(3):717–731
Haack RA, Uzunovic A, Hoover K, Cook JA (2011) Seeking alternatives to probit 9 when developing treatments for wood packaging materials under ISPM No. 15. EPPO Bulletin 41(1): 39–45. http://onlinelibrary.wiley.com/doi/10.1111/epp.2011.41.issue-1/issuetoc. Accessed 23 May 2012
American Society for Testing and Materials (ASTM) (1996) Standard test methods for direct moisture content measurement of wood and wood-base materials. ASTM D 4442-92. ASTM, West Conshohocken, PA
Smith R (1992) Eradication of pinewood nematodes in softwood lumber. In: Proceedings of 13th annual meeting of Canadian Wood Preservation Association. Toronto, Ontario, 3–4 Nov 1992
Acknowledgments
This project was financially supported by USDA-CREES (USDA Methyl Bromide Transitions Program) Grant No. 2009-511-9-05652 to KH and JJ and by FPInnovations industry members, Natural Resources Canada (Canadian Forest Service), and the Provinces of British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Quebec, Nova Scotia, New Brunswick, as well as Newfoundland and Labrador and the Government of Yukon. The authors wish to thank our collaborators Ben Wilson and Glenn Blaker (PSC, Inc) for invaluable discussions and technical support. We also thank Ciprian Lazarescu and Stavros Avramidis (Department of Wood Science, University of British Columbia) for their advice relevant to RF oven use and improvements on heating patterns.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Uzunovic, A., Gething, B., Coelho, A. et al. Lethal temperature for pinewood nematode, Bursaphelenchus xylophilus, in infested wood using radio frequency (RF) energy. J Wood Sci 59, 160–170 (2013). https://doi.org/10.1007/s10086-012-1306-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10086-012-1306-2