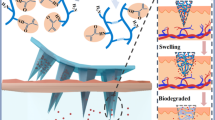
Abstract
Near-infrared light (NIR) triggered transdermal drug delivery systems are of great interest due to their on-demand drug release, which enable to enhance drug treatment efficiency as well as reduce side effect. Herein, a NIR-triggered microneedle (MN) patch array has been fabricated through depositing the photothermal conversion agent and anti-diabetic drug-loaded polymer vesicles with upper critical solution temperature (UCST) into dissolvable polymer matrix. The UCST-type polymer has a clearing point temperature of 41 °C and the drug-loaded polymer vesicles present excellent NIR-triggered and temperature responsive drug release behavior in vitro due to the disassociation of polymer vesicles upon NIR irradiation. After applying MNs to diabetic rats, significant hypoglycemic effect is achieved upon interval NIR irradiation and the blood glucose concentration can decrease to normal state for several hours, which enables to achieve the goal of on-demand drug release. This work suggests that the NIR-triggered MN drug release device has a potential application in the treatment of diabetes, especially for those requiring an active drug release manner.
Similar content being viewed by others
References
Davoodi, P.; Lee, L. Y.; Xu, Q.; Sunil, V.; Sun, Y.; Soh, S.; Wang, C. H. Drug delivery systems for programmed and on-demand release. Adv. Drug Deliv. Rev. 2018, 132, 104–138.
Karimi, M.; Ghasemi, A.; Sahandi Zangabad, P.; Rahighi, R.; Moosavi Basri, S. M.; Mirshekari, H.; Amiri, M.; Shafaei Pishabad, Z.; Aslani, A.; Bozorgomid, M.; Ghosh, D.; Beyzavi, A.; Vaseghi, A.; Aref, A. R.; Haghani, L.; Bahrami, S.; Hamblin, M. R. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 2016, 45, 1457–1501.
Drews, J. Drug discovery: a historical perspective. Science 2000, 287, 1960–1964.
Tibbitt, M. W.; Dahlman, J. E.; Langer, R. Emerging frontiers in drug delivery. J. Am. Chem. Soc. 2016, 138, 704–717.
Fenton, O. S.; Olafson, K. N.; Pillai, P. S.; Mitchell, M. J.; Langer, R. Advances in biomaterials for drug delivery. Adv. Mater. 2018, 30, 1705328.
Gu, X.; Liu, Y.; Chen, G.; Wang, H.; Shao, C.; Chen, Z.; Lu, P.; Zhao, Y. Mesoporous colloidal photonic crystal particles for intelligent drug delivery. ACS Appl. Mater. Interfaces 2018, 10, 33936–33944.
Zhang, H.; Ba, S.; Lee, J. Y.; Xie, J.; Loh, T. P.; Li, T. Cancer biomarker-triggered disintegrable DNA nanogels for intelligent drug delivery. Nano Lett. 2020, 20, 8399–8407.
Meng, X.; Gui, B.; Yuan, D.; Zeller, M.; Wang, C. Mechanized azobenzene-functionalized zirconium metal-organic framework for on-command cargo release. Sci. Adv. 2016, 2, e1600480.
Lu, Y.; Aimetti, A. A.; Langer, R.; Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2017, 2, 16075.
Lavrador, P.; Esteves, M. R.; Gaspar, V. M.; Mano, J. F. Stimuli-responsive nanocomposite hydrogels for biomedical applications. Adv. Funct. Mater. 2021, 31, 2005941.
Liu, G.; Zhao, X.; Zhang, Y.; Xu, J.; Xu, J.; Li, Y.; Min, H.; Shi, J.; Zhao, Y.; Wei, J.; Wang, J.; Nie, G. Engineering biomimetic platesomes for pH-responsive drug delivery and enhanced antitumor activity. Adv. Mater. 2019, 31, 1900795.
Xu, C.; Yan, Y.; Tan, J.; Yang, D.; Jia, X.; Wang, L.; Xu, Y.; Cao, S.; Sun, S. Biodegradable nanoparticles of polyacrylic acid-stabilized amorphous CaCO3 for tunable pH-responsive drug delivery and enhanced tumor inhibition. Adv. Funct. Mater. 2019, 29, 1808146.
Lei, Z.; Ju, Y.; Lin, Y.; Bai, X.; Hu, W.; Wang, Y.; Luo, H.; Tong, Z. Reactive oxygen species synergistic pH/H2O2-responsive of poly(L-lactic acid)-block-poly(sodium 4-styrenesulfonate)/citrate-Fe(III)@ZIF-8 hybrid nanocomposites for controlled drug release. ACS Appl. Bio Mater. 2019, 2, 3648–3658.
Lei, Z.; Tang, Q.; Ju, Y.; Lin, Y.; Bai, X.; Luo, H.; Tong, Z. Block copolymer@ZIF-8 nanocomposites as a pH-responsive multisteps release system for controlled drug delivery. J. Biomater. Sci. Polym. Ed. 2020, 31, 695–711.
Zhou, J.; Tang, Q.; Zhong, J.; Lei, Z.; Luo, H.; Tong, Z.; G. H. Jiang; Liu, X. D. Construction of glucose and H2O2 dual stimuli-responsive polymeric vesicles and their application in controlled drug delivery. J. Mater. Sci. 2018, 53, 14063–14074.
Lin, Y.; Hu, W.; Bai, X.; Ju, Y.; Cao, C.; Zou, S.; Tong, Z.; Cen, C.; Jiang, G.; Kong, X. Glucose- and pH-responsive supramolecular polymer vesicles based on host-guest interaction for transcutaneous delivery of insulin. ACS Appl. Bio Mater. 2020, 3, 6376–6383.
Scott, E. A.; Stano, A.; Gillard, M.; Maio-Liu, A. C.; Swartz, M. A.; Hubbell, J. A. Dendritic cell activation and T cell priming with adjuvant- and antigen-loaded oxidation-sensitive polymersomes. Biomaterials 2012, 33, 6211–6219.
Guo, D. S.; Wang, K.; Wang, Y. X.; Liu, Y. Cholinesterase-responsive supramolecular vesicle. J. Am. Chem. Soc. 2012, 134, 10244–10250.
Wang, H.; Zhang, C.; Zhang, Y.; Tian, R.; Cheng, G.; Pan, H.; Cui, M.; Chang, J. An efficient delivery of photosensitizers and hypoxic prodrugs for a tumor combination therapy by membrane camouflage nanoparticles. J. Mat. Chem. B 2020, 8, 2876–2886.
Yao, C.; Wang, W.; Wang, P.; Zhao, M.; Li, X.; Zhang, F. Near-infrared upconversion mesoporous cerium oxide hollow biophotocatalyst for concurrent pH-/H2O2-responsive O2-evolving synergetic cancer therapy. Adv. Mater. 2018, 30, 1704833.
Moughton, A. O.; O’Reilly, R. K. Thermally induced micelle to vesicle morphology transition for a charged chain end diblock copolymer. Chem. Commun. 2010, 46, 1091–1093.
López-Noriega, A.; Hastings, C. L.; Ozbakir, B.; O’Donnell, K. E.; O’Brien, F. J.; Storm, G.; Hennink, W. E.; Duffy, G. P.; Ruiz-Hernández, E. Hyperthermia-induced drug delivery from thermosensitive liposomes encapsulated in an injectable hydrogel for local chemotherapy. Adv. Healthc. Mater. 2014, 3, 854–859.
Chen, S.; Weitemier, A. Z.; Zeng, X.; He, L.; Wang, X.; Tao, Y.; Huang, A. J. Y.; Hashimotodani, Y.; Kano, M.; Iwasaki, H.; Parajuli, L. K.; Okabe, S.; Teh, D. B. L.; All, A. H.; Tsutsui-Kimura, I.; Tanaka, K. F.; Liu, X.; McHugh, T. J. Near-infrared deep brain stimulation via upconversion nanoparticle-mediated optogenetics. Science 2018, 359, 679.
Hu, W.; Bai, X.; Wang, Y.; Lei, Z.; Luo, H; Tong, Z. Upper critical solution temperature polymer-grafted hollow mesoporous silica nanoparticles for near-infrared-irradiated drug release. J. Mat. Chem. B 2019, 7, 5789–5796.
Wang, J.; Liu, Y.; Ma, Y.; Sun, C.; Tao, W. NIR-activated supersensitive drug release using nanoparticles with a flow core. Adv. Funct. Mater. 2016, 26, 7516–7525.
Qin, J.; Asempah, I.; Laurent, S.; Fornara, A.; Muller, R. N.; Muhammed, M. Injectable superparamagnetic ferrogels for controlled release of hydrophobic drugs. Adv. Mater. 2009, 21, 1354–1357.
Serrà, A.; Gimeno, N.; Gómez, E.; Mora, M.; Lluïsa Sagristá, M.; Vallés, E. Magnetic mesoporous nanocarriers for drug delivery with improved therapeutic efficacy. Adv. Funct. Mater. 2016, 26, 6601–6611.
Mehrali, M.; Thakur, A.; Pennisi, C. P.; Talebian, S.; Arpanaei, A.; Nikkhah, M.; Dolatshahi-Pirouz, A. Nanoreinforced hydrogels for tissue engineering: biomaterials that are compatible with load-bearing and electroactive tissues. Adv. Mater. 2017, 29, 1603612.
Kennedy, S.; Hu, J.; Kearney, C.; Skaat, H.; Gu, L.; Gentili, M.; Vandenburgh, H.; Mooney, D. Sequential release of nanoparticle payloads from ultrasonically burstable capsules. Biomaterials 2016, 75, 91–101.
Di, J.; Yu, J.; Wang, Q.; Yao, S.; Suo, D.; Ye, Y.; Pless, M.; Zhu, Y.; Jing, Y.; Gu, Z. Ultrasound-triggered noninvasive regulation of blood glucose levels using microgels integrated with insulin nanocapsules. Nano Res. 2017, 10, 1393–1402.
Wu, Z.; Lin, X.; Zou, X.; Sun, J.; He, Q. Biodegradable protein-based rockets for drug transportation and light-triggered release. ACS Appl. Mater. Interfaces 2015, 7, 250–255.
Tao, Y.; Chan, H. F.; Shi, B.; Li, M.; Leong, K. W. Light: a magical tool for controlled drug delivery. Adv. Funct. Mater. 2020, 30, 2005029.
Rapp, T. L.; DeForest, C. A. Targeting drug delivery with light: a highly focused approach. Adv. Drug Deliv. Rev. 2021, 171, 94–107.
Alvarez-Lorenzo, C.; Bromberg, L.; Concheiro, A. Light-sensitive intelligent drug delivery systems. Photochem. Photobiol. 2009, 85, 848–860.
Kimura, H.; Lee, C.; Hayashi, K.; Yamauchi, K.; Yamamoto, N.; Tsuchiya, H.; Tomita, K.; Bouvet, M.; Hoffman, R. M. UV light killing efficacy of fluorescent protein-expressing cancer cells in vitro and in vivo. J. Cell. Biochem. 2010, 110, 1439–1446.
Fomina, N.; McFearin, C.; Sermsakdi, M.; Edigin, O.; Almutairi, A. UV and near-IR triggered release from polymeric nanoparticles. J. Am. Chem. Soc. 2010, 132, 9540–9542.
Sponchioni, M.; Bassam, P. R.; Moscatelli, D.; Arosio, P.; Palmiero, U. C. Biodegradable zwitterionic nanoparticles with tunable UCST-type phase separation under physiological conditions. Nanoscale 2019, 11, 16582–16591.
Zou, Y.; Brooks, D. E.; Kizhakkedathu, J. N. A novel functional polymer with tunable LCST. Macromolecules 2008, 41, 5393–5405.
Berndt, I.; Richtering, W. Doubly temperature sensitive core-shell microgels. Macromolecules 2003, 36, 8780–8785.
Seuring, J.; Agarwal, S. First example of a universal and cost-effective approach: polymers with tunable upper critical solution temperature in water and electrolyte solution. Macromolecules 2012, 45, 3910–3918.
Dong, Z.; Mao, J.; Wang, D.; Yang, M.; Wang, W.; Bo, S.; Ji, X. Tunable dual-thermoresponsive phase behavior of zwitterionic polysulfobetaine copolymers containing poly(N,N- dimethylaminoethyl methacrylate)-grafted silica nanoparticles in aqueous solution. Macromol. Chem. Phys. 2014, 215, 111–120.
Xue, X.; Thiagarajan, L.; Braim, S.; Saunders, B. R.; Shakesheff, K.; Alexander, C. Upper critical solution temperature thermo-responsive polymer brushes and a mechanism for controlled cell attachment. J. Mat. Chem. B 2017, 5, 4926–4933.
Maaden, K.; Jiskoot, W.; Bouwstra, J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J. Control. Release 2012, 161, 645–655.
Kim, Y. C.; Park, J. H.; Prausnitz, M. R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568.
Jeong, W. L.; Park, J. H.; Mark, R. P. Dissolving microneedles for transdermal drug delivery. Biomaterials 2008, 29, 2113–2124.
Yu, W.; Jiang, G.; Zhang, Y.; Liu, D.; Xu, B.; Zhou, J. Near-infrared light triggered and separable microneedles for transdermal delivery of metformin in diabetic rats. J. Mat. Chem. B 2017, 5, 9507–9513.
Liu, D.; Yu, B.; Jiang, G.; Yu, W.; Zhang, Y.; Xu, B. Fabrication of composite microneedles integrated with insulin-loaded CaCO3 microparticles and PVP for transdermal delivery in diabetic rats. Mater. Sci. Eng. C 2018, 90, 180–188.
Tong, Z.; Zhou, J.; Zhong, J.; Tang, Q.; Lei, Z.; Luo, H.; Ma, P.; Liu, X. Glucose- and H2O2-responsive polymeric vesicles integrated with microneedle patches for glucose-sensitive transcutaneous delivery of insulin in diabetic rats. ACS Appl. Mater. Interfaces 2018, 10, 20014–20024.
Ye, Y.; Yu, J.; Wang, C.; Nguyen, N. Y.; Walker, G. M.; Buse, J. B.; Gu, Z. Microneedles integrated with pancreatic cells and synthetic glucose-signal amplifiers for smart insulin delivery. Adv. Mater. 2016, 28, 3115–3121.
Xu, B.; Cao, Q.; Zhang, Y.; Yu, W.; Jiang, G. Microneedles integrated with ZnO quantum dots capped mesoporous bioactive glasses for glucose-mediated insulin delivery. ACS Biomater. Sci. Eng. 2018, 4, 2473–2483.
Niskanen, J.; Tenhu, H. How to manipulate the upper critical solution temperature (UCST)?. Polym. Chem. 2017, 8, 220–232.
Xu, Z.; Liu, W. Poly(N-acryloyl glycinamide): a fascinating polymer that exhibits a range of properties from UCST to high-strength hydrogels. Chem. Commun. 2018, 54, 10540–10553.
Acknowledgments
This work was financially supported by the Natural Science Foundation of Zhejiang Province (No. LY20E030005) and the Opening Project of Jiangxi Province Key Laboratory of Polymer Micro/Nano Manufacturing and Devices (No. PMND201905).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Notes
The authors declare no competing financial interest.
Electronic Supplementary Information
10118_2021_2640_MOESM1_ESM.pdf
Polymer Vesicles with Upper Critical Solution Temperature for Near-infrared Light-triggered Transdermal Delivery of Metformin in Diabetic Rats
Rights and permissions
About this article
Cite this article
Hu, W., Su, YW., Jiang, YK. et al. Polymer Vesicles with Upper Critical Solution Temperature for Near-infrared Light-triggered Transdermal Delivery of Metformin in Diabetic Rats. Chin J Polym Sci 40, 157–165 (2022). https://doi.org/10.1007/s10118-021-2640-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10118-021-2640-x