Abstract
This study firstly investigated elemental heterogeneity of municipal solid waste incineration (MSWI) fly ash particles. Two types of heterogeneities were measured quantitatively focusing on three structural components of fly ash particles. They are internal heterogeneity of individual fly ash particles (intra-particle heterogeneity) and inter-particle heterogeneities among fly ash particles. On the surface of fly ash particles, Cl, K, and Na have 0–82% larger intra-particle heterogeneities than Al, Ca, and Si owing to KCl/NaCl-based aggregates. Smaller intra-particle heterogeneity of Ca in semi-soluble component than those of Al and Si suggest that semi-soluble Al/Ca/Si-based matrices around insoluble cores are Ca-based materials including aluminosilicate domains. Inter-particle heterogeneities of Al, Ca, and Si in semi-soluble and insoluble components are 9–40% and 49–352% higher than those of fly ash particle surface, respectively. Inter-particle heterogeneity analysis also suggests that insoluble components mainly consists of Si-, Al-, or Ca-rich cores. MSWI fly ash particles have both internal heterogeneities inside their bodies and also are heterogeneous in inter-particle level. When MSWI fly ash becomes wet during chelate treatment, it changed intra-particle heterogeneity as well as inter-particle heterogeneity. Their variations were contrast depending on element and site (fly ash particle component).




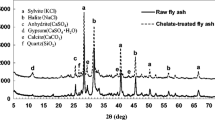
(note: b1 and b2 are referred from Ref. [29])







Similar content being viewed by others
References
Ministry of the Environment (2018) Annual report of waste management in Japan (“Nihon no Haikibutu Shori”) (in Japanese)
Chang FY, Wey MY (2006) Comparison of the characteristics of bottom and fly ashes generated from various incineration processes. J Hazard Mater 138:594–603. https://doi.org/10.1016/j.jhazmat.2006.05.099
Kida A, Noma Y, Imada T (1996) Chemical speciation and leaching properties of elements in municipal incinerator ashes. Waste Manag 16:527–536. https://doi.org/10.1016/S0956-053X(96)00094-3
Song GJ, Kim KH, Seo YC, Kim SC (2004) Characteristics of ashes from different locations at the MSW incinerator equipped with various air pollution control devices. Waste Manag 24:99–106. https://doi.org/10.1016/S0956-053X(03)00073-4
Ecke H, Sakanakura H, Matsuto T et al (2000) State-of-the-art treatment processes for municipal solid waste incineration residues in Japan. Waste Manag Res 18:41–51. https://doi.org/10.1177/0734242X0001800106
Sakai S (1996) Municipal solid waste management in Japan. Waste Manag 16:395–405. https://doi.org/10.1016/S0956-053X(96)00107-9
Mizutani S, van der Sloot HA, Sakai S (2000) Evaluation of treatment of gas cleaning residues from MSWI with chemical agents. Waste Manag 20:233–240. https://doi.org/10.1016/S0956-053X(99)00317-7
Sakanakura H (2007) Formation and durability of dithiocarbamic metals in stabilized air pollution control residue from municipal solid waste incineration and melting processes. Environ Sci Technol 41:1717–1722. https://doi.org/10.1021/es062077e
Jianguo J, Jun W, Xin X et al (2004) Heavy metal stabilization in municipal solid waste incineration flyash using heavy metal chelating agents. J Hazard Mater 113:141–146. https://doi.org/10.1016/j.jhazmat.2004.05.030
Wang FH, Zhang F, Chen YJ et al (2015) A comparative study on the heavy metal solidification/stabilization performance of four chemical solidifying agents in municipal solid waste incineration fly ash. J Hazard Mater 300:451–458. https://doi.org/10.1016/j.jhazmat.2015.07.037
Eighmy TT, Eusden JD, Krzanowski JE et al (1995) Comprehensive approach toward understanding element speciation and leaching behavior in municipal solid waste incineration electrostatic precipitator ash. Environ Sci Technol 29:629–646. https://doi.org/10.1021/es00003a010
Hsiao MC, Wang HP, Wei YL et al (2002) Speciation of copper in the incineration fly ash of a municipal solid waste. J Hazard Mater 91:301–307. https://doi.org/10.1016/S0304-3894(02)00015-8
Hsiao MC, Wang HP, Yang YW (2001) EXAFS and XANES studies of copper in a solidified fly ash. Environ Sci Technol 35:2532–2535. https://doi.org/10.1021/es001374v
Li M, Hu S, Xiang J et al (2003) Characterization of fly ashes from two chinese municipal solid waste incinerators. Energy Fuels 17:1487–1491. https://doi.org/10.1021/ef030092o
Mangialardi T, Paolini AE, Polettini A, Sirini P (1999) Optimization of the solidification/stabilization process of MSW fly ash in cementitious matrices. J Hazard Mater 70:53–70. https://doi.org/10.1016/S0304-3894(99)00132-6
Rémond S, Pimienta P, Bentz D (2002) Effects of the incorporation of municipal solid waste incineration fly ash in cement pastes and mortars: I. Experimental study. Cem Concr Res 32:303–311. https://doi.org/10.1016/S0008-8846(01)00674-3
Rémond S, Bentz D, Pimienta P (2002) Effects of the incorporation of municipal solid waste incineration fly ash in cement pastes and mortars: II: modeling. Cem Concr Res 32:565–576. https://doi.org/10.1016/S0008-8846(01)00722-0
Bayuseno AP, Schmahl WW (2011) Characterization of MSWI fly ash through mineralogy and water extraction. Resour Conserv Recycl 55:524–534. https://doi.org/10.1016/j.resconrec.2011.01.002
Bodénan F, Deniard P (2003) Characterization of flue gas cleaning residues from European solid waste incinerators: assessment of various Ca-based sorbent processes. Chemosphere 51:335–347. https://doi.org/10.1016/S0045-6535(02)00838-X
Le Forestier L, Libourel G (1998) Characterization of flue gas residues from municipal solid waste combustors. Environ Sci Technol 32:2250–2256. https://doi.org/10.1021/es980100t
Kirby CS, Rimstidt JD (1993) Mineralogy and surface properties of municipal solid waste ash. Environ Sci Technol 27:652–660. https://doi.org/10.1021/es00041a008
Nam S, Namkoong W (2012) Irradiation effect on leaching behavior and form of heavy metals in fly ash of municipal solid waste incinerator. J Hazard Mater 199–200:440–447. https://doi.org/10.1016/j.jhazmat.2011.11.049
Raclavská H, Corsaro A, Hartmann-Koval S, Juchelková D (2017) Enrichment and distribution of 24 elements within the sub-sieve particle size distribution ranges of fly ash from wastes incinerator plants. J Environ Manag 203:1169–1177. https://doi.org/10.1016/j.jenvman.2017.03.073
Struis RPWJ, Pasquali M, Borgese L et al (2013) Inertisation of heavy metals in municipal solid waste incineration fly ash by means of colloidal silica—a synchrotron X-ray diffraction and absorption study. RSC Adv 3:14339–14351. https://doi.org/10.1039/c3ra41792a
Weibel G, Eggenberger U, Schlumberger S, Mäder UK (2016) Chemical associations and mobilization of heavy metals in fly ash from municipal solid waste incineration. Waste Manag 62:147–159. https://doi.org/10.1016/j.wasman.2016.12.004
Gilardoni S, Fermo P, Cariati F et al (2004) MSWI fly ash particle analysis by scanning electron microscopy-energy dispersive X-ray spectroscopy. Environ Sci Technol 38:6669–6675. https://doi.org/10.1021/es0494961
Hwang H, Ro C-U (2006) Single-particle characterization of municipal solid waste (MSW) ash particles using low-Z particle electron probe X-ray microanalysis. Atmos Environ 40:2873–2881. https://doi.org/10.1016/j.atmosenv.2006.01.004
Mahieux PY, Aubert JE, Cyr M et al (2010) Quantitative mineralogical composition of complex mineral wastes—contribution of the Rietveld method. Waste Manag 30:378–388. https://doi.org/10.1016/j.wasman.2009.10.023
Kitamura H, Sawada T, Shimaoka T, Takahashi F (2016) Geochemically structural characteristics of municipal solid waste incineration fly ash particles and mineralogical surface conversions by chelate treatment. Environ Sci Pollut Res 23:734–743. https://doi.org/10.1007/s11356-015-5229-5
Kitamura H, Dahlan AV, Tian Y et al (2017) Geochemical form analysis of titanium in chelate-treated municipal solid waste incineration fly ash particles employing correlation analysis of elemental distribution line profiles. J Jpn Soc Civ Eng 73:III_287–III_295. https://doi.org/10.2208/jscejer.73.III_287 (in Japanese)
Kitamura H, Dahlan AV, Tian Y et al (2018) Impact of secondary generated minerals on toxic element immobilization for air pollution control fly ash of a municipal solid waste incinerator. Environ Sci Pollut Res 25:20700–20712. https://doi.org/10.1007/s11356-018-1959-5
Cesur H, Akus Ç (2006) Determination of cadmium and zinc in fertilizer samples by FAAS after solid-phase extraction with freshly precipitated manganese-diethyldhithiocarbamate. Anal Sci 22:727–730. https://doi.org/10.2116/analsci.22.727
Fong C, Sakanakura H, Takahashi F (2015) Mercury immobilization by chelate-complexation for MSWI fly ash: its dependency on chelate/mercury ratio, chelate storage time, and effect of co-existing ions. In: Proceedings of 26th annual conference of the Japan society of materials cycles and waste management, pp 615–616. https://doi.org/10.14912/jsmcwm.26.0_615
Hyks J, Astrup T, Christensen TH (2009) Long-term leaching from MSWI air-pollution-control residues: leaching characterization and modeling. J Hazard Mater 162:80–91. https://doi.org/10.1016/j.jhazmat.2008.05.011
Cappai G, Cara S, Muntoni A, Piredda M (2012) Application of accelerated carbonation on MSW combustion APC residues for metal immobilization and CO2 sequestration. J Hazard Mater 207–208:159–164. https://doi.org/10.1016/j.jhazmat.2011.04.013
Wang L, Jin Y, Nie Y (2010) Investigation of accelerated and natural carbonation of MSWI fly ash with a high content of Ca. J Hazard Mater 174:334–343. https://doi.org/10.1016/j.jhazmat.2009.09.055
Acknowledgements
This study was supported financially by JSPS KAKENHI Grant numbers 26550059, H1504067, 18H01567 and 16J07285. The authors appreciate them greatly.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kitamura, H., Dahlan, A.V., Tian, Y. et al. Intra- and inter-particle heterogeneity of municipal solid waste incineration fly ash particles. J Mater Cycles Waste Manag 21, 925–941 (2019). https://doi.org/10.1007/s10163-019-00853-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-019-00853-1