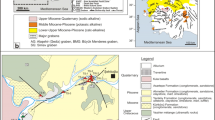
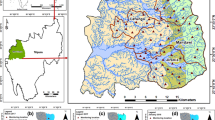
Abstract
Precipitation, surface, and groundwater samples were collected during 2009–2010 in the Sarcheshmeh copper mine drainage basin, Kerman Province, Iran. Groundwater samples were collected from both shallow and deep aquifers. All of the samples were analyzed for stable isotopes, deuterium (2H), and oxygen-18 (18O), and some were analyzed for tritium (3H). The results show a more restricted range of isotopic composition in groundwater samples than in precipitation samples based on the isotopic composition of the precipitation. The isotopic composition of surface and groundwater samples plot to the right of the local meteoric water line of the Sarcheshmeh area and around the evaporation line, indicating that the groundwater within the study area originates from meteoric water that has undergone secondary evaporation before or during recharge. Tritium was below the detection limit in the deep groundwater samples while shallow groundwater samples had tritium concentrations between 1.2 and 1.7 TU, which indicates a longer residence time for deep groundwater.
Resumen
Se colectaron muestras de aguas subterráneas de superficie y de precipitación durante 2009–2010 en la cuenca de drenaje de la mina de cobre Sarcheshmeh, Provincia de Kermán, Irán. Se recolectaron muestras de aguas subterráneas desde acuíferos profundos y superficiales. En todas las muestras se analizaron isótopos estables, deuterio (2H) y oxígeno18 (18O) y en algunas se analizó tritio (3H). Los resultados muestran una composición isotópica en las muestras subterráneas en un rango más restringido que en las muestras de precipitación basadas en la composición isotópica de esta última. La composición isotópica de las muestras de agua subterráneas y de superficie caen a la derecha de la línea de agua meteórica local (LMWL) del área de Sarcheshmeh y alrededor de la línea de evaporación indicando que el agua subterránea dentro del área de estudio se origina desde agua meteórica que sufre una evaporación secundaria antes o durante la recarga. El tritio estuvo por debajo del límite de detección en las muestras de agua subterránea profunda mientras que las muestras de agua subterránea superficial tenían concentraciones de tritio entre 1,2 y 1,7 TU, lo que indica un largo tiempo de residencia para el agua subterránea profunda.
抽象
于2009–2010年间,在伊朗 克尔曼省(Kerman)的Sarcheshmeh铜矿区采集了大气降水、地表水和地下水水样。地下水水样分别取自浅层和深层含水层。所有水样都进行了稳定同位素氘(2H)和 18O测试,部分水样进行了氚(3H)分析。试验结果表明,地下水同位素组份范围比大气降水更窄。地表水和地下水样同位素组成分布于Sarcheshmeh地区当地降水线(LMWL)的右侧和蒸发线周围,表明研究区域内地下水来源于降水,且降水在补给地下水之前或补给过程中经受了第二次蒸发。深层地下水样的氚浓度低于检测极限,而浅层地下水样的氚浓度在1.2至1.7 TU 之间,表明深层地下水的滞留时间更长。











Similar content being viewed by others
References
Abbott MD, Lini A, Bierman PR (2000) δ18O, δD and 3H measurements constrain groundwater recharge patterns in an upland fractured bedrock aquifer Vermont USA. J Hydrol 228:101–112
Abu-Jaber N, Kharabsheh A (2008) Ground water origin and movement in the upper Yarmouk basin, northern Jordan. Environ Geol 54:1355–1365
Acheampong SY, Hess JW (2000) Origin of the shallow groundwater system in the southern Voltaian Sedimentary Basin of Ghana: an isotopic approach. J Hydrol 233:37–53
Axel H (2006) Use of stable and radioactive isotopes and gaseous tracers for estimating groundwater recharge time of residence mixing of the different types of groundwater and origin in the Silao Romita aquifer Guanajuato central Mexico. Freiberg Online Geol, vol 17
Chen Z, Nie Z, Zhang G, Wan L, Shen J (2006) Environmental isotopic study on the recharge and residence time of groundwater in the Heihe River Basin northwestern China. Hydrogeol J 14:1635–1651
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. Lewis Publication, Boca Raton
Cloutier V, Lefebvre R, Savard R, Bourque E, Therrien R (2006) Hydrogeochemistry and groundwater origin of the Basses-Laurentides sedimentary rock aquifer system St Lawrence Lowlands Quebec Canada. Hydrogeol J 14:573–590
Craig H (1961) Isotopic variations in meteoric waters. Science 133:1702–1703
Drimme RJ, Shouakar-Stash O, Walters R, Heemskerk AR (2001) Hydrogen isotope ratio of H2O by automatic elemental analysis-continuous flow-isotope ratio mass spectrometry. Technical procedure 4.1, Rev 00. Environmental Isotope Laboratory, Department of Earth Sciences, University of Waterloo, Canada
Edmunds WM, Darling WG, Kinniburgh DG, Kotoub S, Mahgoub S (1992) Sources of recharge at Abu Delaig, Sudan. J Hydrol 131:1–24
Engle MA, Goff F, Jewett DG, Reller GJ, Bauman JB (2008) Application of environmental groundwater tracers at the Sulphur Bank Mercury Mine, California, USA. Hydrogeol J 16:559–573
Epstein S, Mayeda T (1953) Variation of 18O content of waters from natural sources. Geochim Cosmochim Acta 4:213–224
Fantong WY, Satake H, Aka FT, Ayonghe SN, Asai K, Mandal AK, Ako AA (2010) Hydrochemical and isotopic evidence of recharge apparent age and flow direction of groundwater in the Mayo Tsanaga River Basin Cameroon: bearings on contamination. Environ Earth Sci 60:107–120
Faure G (1986) Principles of isotope geology. Wiley, New York
Friedman I, Machta L, Soiler R (1962) Water-vapor exchange between a water droplet and its environment. J Geophys Res 67:2761–2766
Gat JR (1971) Comments on the stable isotope method in regional groundwater investigations. Water Resour Res 7:980–993
Gat JR, Carmi I (1970) Evolution of the isotopic composition of atmospheric waters in the Mediterranean Sea area. J Geophys Res 75:3039–3048
Geirnaert W, Groenand M, Van der Sommen J (1984) Isotope studies as a final stage in groundwater investigations on the African shield. In: Challenges in African hydrology and water resources. IAHS publication, Harare, no 144, pp 141–153
Girard P, Hillaire-Marcel C, Oga MS (1997) Determining the recharge mode of sahelian aquifers using water isotopes. J Hydrol 197:189–202
Goni BI (2006) Tracing stable isotope values from meteoric water to groundwater in the southwestern part of the Chad basin. Hydrogeol J 14:742–752
Gupta H, Ray S (2007) Geothermal energy: an alternative resource for the 21st century. Elsevier, Amsterdam
Hamed Y, Dassi L, Tarki M, Ahmadi R, Mehdi K, Ben Dhia H (2011) Groundwater origins and mixing pattern in the multilayer aquifer system of the Gafsa-south mining district: a chemical and isotopic approach. Environ Earth Sci 63:1355–1368
Kazemi GA, Lehr JH, Perrochet P (2006) Groundwater age. Wiley-Interscience, New York
Khademi H, Mermut AR, Krouse HR (1997) Isotopic composition of gypsum hydration water in selected landforms from central Iran. Chemical Geol 138:245–255
Lee KS, Wenner DB, Lee I (1999) Using H and O isotopic data for estimating the relative contributions of rainy and dry season precipitation to groundwater: example from Cheju Island Korea. J Hydrol 222:65–74
Leontiadis IL, Vergis S, Christodoulou TH (1996) Isotope hydrology study of areas in Eastern Macedonia and Thrace, Northern Greece. J Hydrol 182:1–17
Leybourne MI, Goodfellow WD (2007) Br/Cl ratios and O, H, C and B isotopic constraints on the origin of saline waters from eastern Canada. Geochim Cosmochim Acta 71:2209–2223
Leybourne MI, Clark ID, Goodfellow WD (2006) Stable isotope geochemistry of ground and surface waters associated with undisturbed massive sulfide deposits; constraints on origin of waters and water–rock reactions. Chemical Geol 231:300–325
Matter JM, Waber HN, Loew S, Matter A (2005) Recharge areas and geochemical evolution of groundwater in an alluvial aquifer system in the Sultanate of Oman. Hydrogeol J 14:203–224
Mohammadi Z (2006) Method of leakage study at karst dam sites, Zagros zone, Iran. PhD Thesis, University of Shiraz, Iran
Mohammadzadeh H (2010) The meteoric relationship for18O and 2H in precipitations and isotopic compositions of water resources in Mashhad area (NE Iran). In: Proceeding of 1st international applied geological congress, Mashhad, Iran
Mohammadzadeh H, Ebrahimpoor S (2012) Application of stable isotope and hydrogeochemistry to investigate the origin and water resources quality variations in Zarivar lake catchment area. J Water Soil 26(4):1018–1041 (in Persian)
Morton KL, Mekerk FA (1993) A phased approach to mine dewatering. Mine Water Environ 12:27–34
Njitchoua R, Dever L, Fontes JC, Naah E (1997) Geochemistry origin and recharge mechanisms of groundwaters from the Garoua Sandstone aquifer, northern Cameroon. J Hydrol 190:123–140
Ortega-Guerrero A (2003) Origin and geochemical evolution of groundwater in a closed-basin clayey aquitard, northern Mexico. J Hydrol 284:26–44
Rose TP, Davisson ML, Hudson GB, Varian AR (1997) Environmental isotope investigation of groundwater flow in the Honey Lake basin, California and Nevada. Isotope Sciences Div, Lawrence Livermore National Lab, Livermore, CA, USA
Sahraei Parizi H, Samani N (2012) Geochemical evolution and quality assessment of water resources in the Sarcheshmeh copper mine area (Iran) using multivariate statistical techniques. Environ Earth Sci 69(5):1699–1718
Shahabpour J (1982) Aspects of alteration and mineralization at the Sarcheshmeh copper-molybdenum deposit, Kerman, Iran. PhD thesis, Leeds University, UK
Shahabpour J, Kramers JD (1987) Lead isotope data from the Sarcheshmeh porphyry copper deposit, Kerman, Iran. Miner Depos 22:278–281
SRK (2011) Sarcheshmeh copper mine open pit design; main report. SRK Consulting Ltd, Wales
Wassenaar LI, Athanasopoulos P, Hendry MJ (2011) Isotope hydrology of precipitation, surface and ground waters in the Okanagan Valley, British Columbia, Canada. J Hydrol 411(1):37–48
Acknowledgments
The authors thank the National Iranian Copper Industries Co. for funding this work. Part of this research was carried out when the second author visited the University of Waterloo, Canada. Constructive comments given by the Editor-in-Chief, the Associate Editor (Led Murray), and two anonymous reviewers are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Parizi, H.S., Samani, N. Environmental Isotope Investigation of Groundwater in the Sarcheshmeh Copper Mine Area, Iran. Mine Water Environ 33, 97–109 (2014). https://doi.org/10.1007/s10230-014-0277-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-014-0277-5