Abstract
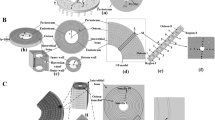
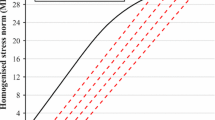
The underlying mechanisms by which bone cells respond to mechanical stimuli or how mechanical loads act on osteocytes housed in lacunae in bone are not well understood. In this study, a multilevel finite element (FE) approach is applied to predict local cell deformations in bone tissue. The local structure of the matrix dictates the local mechanical environment of an osteocyte. Cell deformations are predicted from detailed linear FE analysis of the microstructure, consisting of an arrangement of cells embedded in bone matrix material. This work has related the loads applied to a whole femur during the stance phase of the gait cycle to the strain of a single lacuna and of canaliculi. The predicted bone matrix strains around osteocyte lacunae and canaliculi were nonuniform and differed significantly from the macroscopically measured strains. Peak stresses and strains in the walls of the lacuna were up to six times those in the bulk extracellular matrix. Significant strain concentrations were observed at sites where the process meets the cell body.
Similar content being viewed by others
References
Adachi T, Sato K, Tomita Y (2003) Directional dependence of osteoblastic calcium response to mechanical stimuli. Biomech Model Mechanobiol 2(2):73–82
Ashman RB, Cowin SC, Van Buskirk WC, Rice JC (1984) A continuous wave technique for the measurement of the elastic properties of cortical bone. J Biomech 17:349–361
Brand RA, Stanford CM, Nicolella DP (2001) Primary adult human bone do not respond to tissue (continuum) level strains. J Orthop Sci 6:295–301
Breuls RGM, Sengers BG, Oomens CWJ, Bouten CVC, Baaijens FPT (2002) Predicting local cell deformations in engineered tissue constructs:a multilevel finite element approach. Trans ASME 124:198–207
Brighton CT, Fisher JR Jr, Levine SE, et al. (1996) The biochemical pathway mediating the proliferative response of bone cells to a mechanical stimulus. J Bone Joint Surg Am 78(9):1337–1347
Burr DB, Robling AG, Turner CH (2002) Effects of biomechanical stress on bones in animals. Bone 30:781–786
Cheung G, Zalzal P, Bhandari M, Spelt JK, Papini M (2004) Finite element analysis of a femoral retrograde intramedullary nail subject to gait loading. Med Eng Phys 26:93–108
Cowin SC, Moss-Salentijn L, Moss ML (1991) Candidates for the mechanosensory system in bone. J Biomech Eng 115:191–197
Deligianni DD, Tanner KE, Missirlis YF, Bonfield W (1991) Mechanical behavior of trabecular bone of the human femoral head in females. J Mat Sci Mater Med 2:168–175
Duda GN, Heller M, Albinger J, et al. (1998) Influence of muscle forces on femoral strain distribution. J Biomech 31:841–846
Gardner TN, Stoll T, Marks L, Mishra, Knothe Tate M (2000) The influence of mechanical stimulus on the pattern of tissue differentiation in a long bone fracture-an FEM study. J Biomech 33:415–425
Guilak F, Mow VC (2000) The mechanical environment of the chondrocyte:a biphasic finite element model of cell-matrix interactions in articular cartilage. J Biomech 33(12):1663–1673
Gururaja S, Kim HJ, Swan CC, Brand RA, Lakes RS (2005) Modeling deformation-induced fluid flow in cortical bone’s canalicular-lacunar system. Ann Biomed Eng 33(1):7–25
Kaspar D, Seidl W, Neidlinger-Wilke C, Claes L (2000) In vitro effects of dynamic strain on the proliferative and metabolic activity of human osteoblasts. J Musculoskelet Neuronal Interact 1(2):161–164
Lanyon LE, Hampson WE Jr, Goodship AE, Shah JS (1975) Bone deformation recorded in vivo from strain gauges attached to the human tibial shaft. Acta Orthop Scand 46:256–268
Liebschner MAK, Keller TS (2005) Hydraulic strengthening affects the stiffness and strength of cortical bone. Ann Biomed Eng 33(1):26–38
Mak AFT, Zhang JD (2001) Numerical simulation of streaming potentials due to deformation-induced hierarchical flows in cortical bone. J Biomech Eng 123:66–70
Mak AFT, Huang DT, Zhang JD, Tong P (1997) Deformation-induced hierarchical flows and drag forces in bone canaliculi and matrix microporosity. J Biomech 30(1):11–18
McGarry JG, Klein-Nulend J, Mullender MG, Prendergast PJ (2005) A comparison of strain and fluid shear stress in stimulating bone cell responses: a computational and experimental study. FASEB J 19(3):482–484
Mullender M, El Haj AJ, Yang Y, van Duin MA, Burger EH, Klein-Nulend J (2004) Mechanotransduction of bone cells in vitro:mechanobiology of bone tissue. Med Biol Eng Comput 42:14–21
Nicolella DP, Lankford J (2002) Microstructural strain near osteocyte lacuna in cortical bone in vitro. J Musculoskelet Neuronal Interact 2(3):261–263
Owan I, Burr DB, Turner CH, et al. (1997) Mechanotransduction in bone:osteoblasts are more responsive to fluid forces than mechanical strain. Am J Physiol 273:C810-C815
Piekarski K, Munro M (1977) Transport mechanism operating between blood supply and osteocytes in long bones. Nature 269:80–82
Qin Y-X, Lin W, Rubin C (2002) The pathway of bone fluid flow as defined by in vivo intramedullary pressure and streaming potential measurements. J Biomed Eng 30:693–702
Reilly GC (2003) Observations of microdamage around osteocyte launae in bone. J Biomech 33:1131–1334
Reilly DT, Burnstein AH, Frankel VH (1974) The elastic modulus for bone. J Biomech 7:271–275
Swan CC, Lakes RS, Brand RA, Stewart KJ (2003) Micromechanically based poroelastic modeling of fluid flow in Haversian bone. J Biomech Eng 125(1):25–37
Taddei F, Cristofolini L, Martelli S, Gill HS, Viceconti M (2006) Subject specific finite element models of long bones:An in vitro evaluation of the overall accuracy. J Biomech 39(13):2457–2467
Tang LL, Wang YL, Pan J, Cai SX (2004) The effect of step-wise increased stretching on rat calvarial osteoblast collagen production. J Biomech 37(1):157–1661
Taylor ME, Tanner KE, Freeman MAR, Yettram AL (1996) Stress and strain distribution within the intact femur:compression or bending? Med Eng Phys 18(2):122–131
Turner CH, Anne V, Pidaparti RM (1997) A uniform strain criterion for trabecular bone adaptation:do continuum-level strain gradients drive adaptation? J Biomech 30:555–563
Viceconti M, Davinelli M, Taddei F, Cappello A (2004) Automatic generation of accurate subject-specific bone finite element models to be used in clinical studies. J Biomech 17:1597–1605
Wang L, Fritton SP, Cowin SC, Weinbaum S (1999) Fluid pressure relaxation depends upon osteonal microstructure:modeling an oscillatory bending experiment. J Biomech 32:663–672
Weinbaum S, Cowin SC, Zeng Y (1994) A model for the excitation of osteocytes by mechanical loadind — induced bone fluid shear stresses. J Biomech 27(3):339–360
Wirtz DC, Schiffers N, Pandorf T, Radermacher K, Weichert D, Forst R (2000) Critical evaluation of known bone material properties to realize anisotropic FE-simulation of the proximal femur. J Biomech 33(10):1325–1330
You J, Yellowley CE, Donahue HJ, et al. (2000) Substrate deformation levels associated with routine physical activity are less stimulatory to bone cells relative to loading-induced oscillatory fluid flow. J Biomech Eng 122:387–393
You J, Reilly GC, Zhen X, Yellowley CE, Chen Q, Donahue HJ, Jacobs CR (2001) Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. J Biol Chem 276:13365–13371
Zhang DS, Weinbaum S, Cowin SC (1998) Estimates of the peak pressures in bone pore water. J Biomech Eng 120(6):697–703
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deligianni, D.D., Apostolopoulos, C.A. Multilevel finite element modeling for the prediction of local cellular deformation in bone. Biomech Model Mechanobiol 7, 151–159 (2008). https://doi.org/10.1007/s10237-007-0082-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-007-0082-1