Abstract
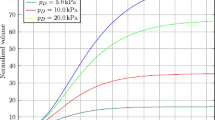
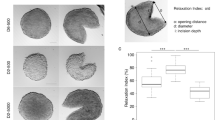
Tumor spheroids constitute an effective in vitro tool to investigate the avascular stage of tumor growth. These three-dimensional cell aggregates reproduce the nutrient and proliferation gradients found in the early stages of cancer and can be grown with a strict control of their environmental conditions. In the last years, new experimental techniques have been developed to determine the effect of mechanical stress on the growth of tumor spheroids. These studies report a reduction in cell proliferation as a function of increasingly applied stress on the surface of the spheroids. This work presents a specialization for tumor spheroid growth of a previous more general multiphase model. The equations of the model are derived in the framework of porous media theory, and constitutive relations for the mass transfer terms and the stress are formulated on the basis of experimental observations. A set of experiments is performed, investigating the growth of U-87MG spheroids both freely growing in the culture medium and subjected to an external mechanical pressure induced by a Dextran solution. The growth curves of the model are compared to the experimental data, with good agreement for both the experimental settings. A new mathematical law regulating the inhibitory effect of mechanical compression on cancer cell proliferation is presented at the end of the paper. This new law is validated against experimental data and provides better results compared to other expressions in the literature.









Similar content being viewed by others
References
Ambrosi D, Mollica F (2004) The role of stress in the growth of a multicell spheroid. J Math Biol 48:477–499
Ambrosi D, Preziosi L (2009) Cell adhesion mechanisms and stress relaxation in the mechanics of tumours. Biomech Model Mechanobiol 8:397–413
Ambrosi D, Preziosi L, Vitale G (2012) The interplay between stress and growth in solid tumors. Mech Res Commun 42:87–91
Baumgartner W, Hinterdorfer P, Ness W et al (2000) Cadherin interaction probed by atomic force microscopy. Proc Natl Acad Sci USA 97:4005–4010
Bonnet-Gonnet C, Belloni L, Cabane B (1994) Osmotic pressure of latex dispersions. Langmuir 10:4012–4021
Bouchoux A, Cayemitte PE, Jardin J et al (2009) Casein micelle dispersions under osmotic stress. Biophys J 96:693–706
Byrne H, Preziosi L (2003) Modelling solid tumour growth using the theory of mixtures. Math Med Biol 20:341–366
Carlsson J (1977) A proliferation gradient in three-dimensional colonies of cultured human glioma cells. Int J Cancer 20:129–136
Carlsson J, Yuhas JM (1984) Liquid-overlay culture of cellular spheroids. Recent Results Cancer Res 95:1–23
Casciari JJ, Sotirchos SV, Sutherland RM (1992a) Mathematical modelling of microenvironment and growth in EMT6/Ro multicellular tumour spheroids. Cell Prolif 25:1–22
Casciari JJ, Sotirchos SV, Sutherland RM (1992b) Variations in tumor cell growth rates and metabolism with oxygen concentration, glucose concentration, and extracellular pH. J Cell Physiol 151:386–394
Chaplain MAJ, Graziano L, Preziosi L (2006) Mathematical modelling of the loss of tissue compression responsiveness and its role in solid tumour development. Math Med Biol 23:197–229
Chauhan VP, Jain RK (2013) Strategies for advancing cancer nanomedicine. Nat Mater 12:958–62
Cheng G, Tse J, Jain RK, Munn LL (2009) Micro-environmental mechanical stress controls tumor spheroid size and morphology by suppressing proliferation and inducing apoptosis in cancer cells. PLoS One 4:e4632
Ciarletta P, Ambrosi D, Maugin GA, Preziosi L (2013a) Mechano-transduction in tumour growth modelling. Eur Phys J E 36:23
Ciarletta P, Preziosi L, Maugin GA (2013b) Mechanobiology of interfacial growth. J Mech Phys Solids 61:852–872
Delarue M, Montel F, Vignjevic D et al (2014) Compressive stress inhibits proliferation in tumor spheroids through a volume limitation. Biophys J 107:1821–1828
Desmaison A, Frongia C, Grenier K et al (2013) Mechanical stress impairs mitosis progression in multi-cellular tumor spheroids. PLoS One 8:e80447
Dorie MJ, Kallman RF, Rapacchietta DF et al (1982) Migration and internalization of cells and polystyrene microsphere in tumor cell spheroids. Exp Cell Res 141:201–209
Ehlers W, Markert B, Rohrle O (2009) Computational continuum biomechanics with application to swelling media and growth phenomena. GAMM-Mitteilungen 32:135–156. doi:10.1002/gamm.200910013
Fernández-Sánchez ME, Barbier S, Whitehead J et al (2015) Mechanical induction of the tumorigenic \(\upbeta \)-catenin pathway by tumour growth pressure. Nature 523:92–95
Folkman J, Hochberg M (1973) Self-regulation of growth in three dimensions. J Exp Med 138:745–753
Freyer JP, Sutherland RM (1986) Regulation of growth saturation and development of necrosis in EMT6/Ro multicellular spheroids by the glucose and oxygen supply. Cancer Res 46:3504–3512
Friedrichs J, Legate KR, Schubert R et al (2013) A practical guide to quantify cell adhesion using single-cell force spectroscopy. Methods 60:169–78
Galle J, Preziosi L, Tosin A (2009) Contact inhibition of growth described using a multiphase model and an individual cell based model. Appl Math Lett 22:1483–1490
Giverso C, Scianna M, Grillo A (2015) Growing avascular tumours as elasto-plastic bodies by the theory of evolving natural configurations. Mech Res Commun 68:31–39
Grantab R, Sivananthan S, Tannock IF (2006) The penetration of anticancer drugs through tumor tissue as a function of cellular adhesion and packing density of tumor cells. Cancer Res 66:1033–9
Gray WG, Miller CT (2005) Thermodynamically constrained averaging theory approach for modeling flow and transport phenomena in porous medium systems: 1. Motivation and overview. Adv Water Resour 28:161–180
Gray WG, Miller CT (2014) Introduction to the thermodynamically constrained averaging theory for porous medium systems, 1st edn. Springer International Publishing, Berlin
Greenspan HP (1976) On the growth and stability of cell cultures and solid tumors. J Theor Biol 56:229–42
Helenius J, Heisenberg C-P, Gaub HE, Muller DJ (2008) Single-cell force spectroscopy. J Cell Sci 121:1785–91
Helmlinger G, Netti PA, Lichtenbeld HC et al (1997) Solid stress inhibits the growth of multicellular tumor spheroids. Nat Biotechnol 15:778–783
Jain RK (1988) Determinants of tumor blood flow: a review. Cancer Res 48:2641–2658
Jain RK, Martin JD, Stylianopoulos T (2014) The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng 16:321–46
Jain RK, Stylianopoulos T (2010) Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol 7:653–64
Kaufman LJ, Brangwynne CP, Kasza KE et al (2005) Glioma expansion in Collagen I matrices: analyzing collagen concentration-dependent growth and motility patterns. Biophys J 89:635–650
Kim T-H, Mount CW, Gombotz WR, Pun SH (2010) The delivery of doxorubicin to 3-D multicellular spheroids and tumors in a murine xenograft model using tumor-penetrating triblock polymeric micelles. Biomaterials 31:7386–97
Kim Y, Stolarska MA, Othmer HG (2011) The role of the microenvironment in tumor growth and invasion. Prog Biophys Mol Biol 106:353–379
Leder K, Pitter K, Laplant Q et al (2014) Mathematical modeling of PDGF-driven glioblastoma reveals optimized radiation dosing schedules. Cell 156:603–16
Lewis RW, Schrefler BA (1998) The finite element method in the static and dynamic deformation and consolidation of porous media. Wiley, New York
Longo D, Fauci A, Kasper D, Hauser S (2011) Harrison’s principles of internal medicine. McGraw-Hill Professional, New York
Lowengrub JS, Frieboes HB, Jin F et al (2010) Nonlinear modelling of cancer: bridging the gap between cells and tumours. Nonlinearity 23:R1–R91
Michor F, Liphardt J, Ferrari M, Widom J (2011) What does physics have to do with cancer? Nat Rev Cancer 11:657–70
Mikhail AS, Eetezadi S, Allen C (2013) Multicellular tumor spheroids for evaluation of cytotoxicity and tumor growth inhibitory effects of nanomedicines in vitro: A comparison of Docetaxel-loaded block copolymer micelles and Taxotere\(\textregistered \). PLoS One 8:e62630
Montel F, Delarue M, Elgeti J et al (2011) Stress clamp experiments on multicellular tumor spheroids. Phys Rev Lett 107:188102
Montel F, Delarue M, Elgeti J et al (2012) Isotropic stress reduces cell proliferation in tumor spheroids. New J Phys 14:055008
Mpekris F, Angeli S, Pirentis AP, Stylianopoulos T (2015) Stress-mediated progression of solid tumors: effect of mechanical stress on tissue oxygenation, cancer cell proliferation, and drug delivery. Biomech Model Mechanobiol 14:1391–1402
Mueller-Klieser W (1986) Influence of glucose and oxygen supply conditions on the oxygenation of multicellular spheroids. Br J Cancer 345–353
Mueller-Klieser W, Sutherland R (1982) Oxygen tensions in multicellspheroids of two cell lines. Br J Cancer 256–264
Netti PA, Berk DA, Swartz MA et al (2000) Role of extracellular matrix assembly in interstitial transport in solid tumors Cancer Res 60:2497–2503
Pinder GF, Gray WG (2008) Essentials of multiphase flow in porous media, 1st edn. Wiley, New York
Preziosi L, Tosin A (2009a) Multiphase and multiscale trends in cancer modelling. Math Model Nat Phenom 4:1–11
Preziosi L, Tosin A (2009b) Multiphase modelling of tumour growth and extracellular matrix interaction: mathematical tools and applications. J Math Biol 58:625–656
Preziosi L, Vitale G (2011) A multiphase model of tumor and tissue growth including cell adhesion and plastic reorganization. Math Model Methods Appl Sci 21:1901–1932
Puech P-H, Taubenberger A, Ulrich F et al (2005) Measuring cell adhesion forces of primary gastrulating cells from zebrafish using atomic force microscopy. J Cell Sci 118:4199–206
Roose T, Chapman SJ, Maini PK (2007) Mathematical models of avascular tumor growth. SIAM Rev 49:179–208
Roose T, Netti PA, Munn LL et al (2003) Solid stress generated by spheroid growth estimated using a linear poroelasticity model. Microvasc Res 66:204–212
Sciumè G, Gray WG, Hussain F et al (2013a) Three phase flow dynamics in tumor growth. Comput Mech 53:465–484
Sciumè G, Santagiuliana R, Ferrari M et al (2014) A tumor growth model with deformable ECM. Phys Biol 11:65004
Sciumè G, Shelton S, Gray WG et al (2013b) A multiphase model for three-dimensional tumor growth. New J Phys 15:015005
Stigliano C, Key J, Ramirez M et al (2015) Radiolabeled polymeric nanoconstructs loaded with Docetaxel and Curcumin for cancer combinatorial therapy and nuclear imaging. Adv Funct Mater 25:3371–3379
Sutherland R, Carlsson J, Durand R, Yuhas J (1981) Spheroids in cancer research. Cancer Res 41:2980–2984
Sutherland RM, McCredie JA, Inch WR (1971) Growth of multicell spheroids in tissue culture as a model of nodular carcinomas. J Natl Cancer Inst 46:113–120
Vinci M, Gowan S, Boxall F et al (2012) Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol 10:29
Wise SM, Lowengrub JS, Frieboes HB, Cristini V (2008) Three-dimensional multispecies nonlinear tumor growth-I. Model and numerical method. J Theor Biol 253:524–543
Acknowledgments
The research that lead to the present paper was partially supported by a grant of the group GNFM of INdAM.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mascheroni, P., Stigliano, C., Carfagna, M. et al. Predicting the growth of glioblastoma multiforme spheroids using a multiphase porous media model. Biomech Model Mechanobiol 15, 1215–1228 (2016). https://doi.org/10.1007/s10237-015-0755-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-015-0755-0