Abstract
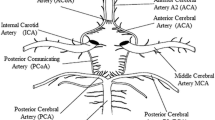
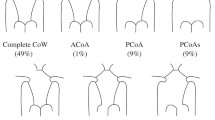
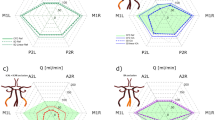
The hemodynamic alteration in the cerebral circulation caused by the geometric variations in the cerebral circulation arterial network of the circle of Wills (CoW) can lead to fatal ischemic attacks in the brain. The geometric variations due to impairment in the arterial network result in incomplete cerebral arterial structure of CoW and inadequate blood supply to the brain. Therefore, it is of great importance to understand the hemodynamics of the CoW, for efficiently and precisely evaluating the status of blood supply to the brain. In this paper, three-dimensional computational fluid dynamics of the main CoW vasculature coupled with zero-dimensional lumped parameter model boundary condition for the CoW outflow boundaries is developed for analysis of the blood flow distribution in the incomplete CoW cerebral arterial structures. The geometric models in our study cover the arterial segments from the aorta to the cerebral arteries, which can allow us to take into account the innate patient-specific resistance of the arterial trees. Numerical simulations of the governing fluid mechanics are performed to determine the CoW arterial structural hemodynamics, for illustrating the redistribution of the blood flow in CoW due to the structural variations. We have evaluated our coupling methodology in five patient-specific cases that were diagnosed with the absence of efferent vessels or impairment in the connective arteries in their CoWs. The velocity profiles calculated by our approach in the segments of the patient-specific arterial structures are found to be very close to the Doppler ultrasound measurements. The accuracy and consistency of our hemodynamic results have been improved (to \(16.1 \pm 18.5\) %) compared to that of the pure-resistance boundary conditions (of 43.5 \(\pm \) 28 %). Based on our grouping of the five cases according to the occurrence of unilateral occlusion in vertebral arteries, the inter-comparison has shown that (i) the flow reduction in posterior cerebral arteries is the consequence of the unilateral vertebral arterial occlusion, and (ii) the flow rate in the anterior cerebral arteries is correlated with the posterior structural variations. This study shows that our coupling approach is capable of providing comprehensive information of the hemodynamic alterations in the pathological CoW arterial structures. The information generated by our methodology can enable evaluation of both the functional and structural status of the clinically significant symptoms, for assisting the treatment decision-making.











Similar content being viewed by others
Abbreviations
- ACA:
-
Anterior cerebral artery
- MCA:
-
Middle cerebral artery
- PCA:
-
Posterior cerebral artery
- PCoA:
-
Posterior communication artery
- ACoA:
-
Anterior communication artery
- ICA:
-
Internal carotid artery
- ECA:
-
External carotid artery
- CCA:
-
Common carotid artery
- VA:
-
Vertebral artery
- L_:
-
Left
- R_:
-
Right
- PSV:
-
Peak systolic velocity
- LPM:
-
Lump parameter model
References
AbuRahma AF, Bandyk DF (2013) Noninvasive vascular diagnosis: a practical guide to therapy, 3rd edn. Springer, Berlin
Alastruey J, Moore SM, Parker KH, David T, Peiró J, Sherwin SJ (2008) Reduced modelling of blood flow in the cerebral circulation: coupling 1-D, 0-D and cerebral autoregulation models. Int J Numer Methods Fluids 56(8):1061–1067
Alastruey J, Parker KH, Peiro J, Byrd SM, Sherwin SJ (2007) Modelling the circle of Willis to assess the effects of anatomical variations and occlusions on cerebral flows. J Biomech 40(8):1794–1805
Anthea M, Hopkins J, McLaughlin CW, Johnson S, Warner MQ, LaHart D, Wright JD (1999) Human biology and health. Prentice Hall, New Jersey
Arenillas JF, Molina CA, Montaner J, Abilleira S, González-Sánchez MA, Álvarez-Sabín J (2001) Progression and clinical recurrence of symptomatic middle cerebral artery stenosis: a long-term follow-up transcranial Doppler ultrasound study. Stroke 32(12):2898–2904
Borazjani I, Ge L, Le T, Sotiropoulos F (2013) A parallel overset-curvilinear-immersed boundary framework for simulating complex 3D incompressible flows. Comput Fluids 77:76–96
Cassot F, Zagzoule M, Marc-Vergnes JP (2000) Hemodynamic role of the circle of Willis in stenoses of internal carotid arteries: an analytical solution of a linear model. J Biomech 33(4):395–405
Chaichana T, Sun Z, Jewkes J (2011) Computation of hemodynamics in the left coronary artery with variable angulations. J Biomech 44(10):1869–1878
Cieslicki K, Ciesla D (2005) Investigations of flow and pressure distributions in physical model of the circle of Willis. J Biomech 38(11):2302–2310
Coogan J, Humphrey J, Figueroa CA (2013) Computational simulations of hemodynamic changes within thoracic, coronary, and cerebral arteries following early wall remodeling in response to distal aortic coarctation. Biomech Model Mechanobiol 12:79–93
de Boorder MJ, van der Grond J, van Dongen AJ, Klijn CJ, Kappelle LJ, Van Rijk PP, Hendrikse JS (2006) Measurements of regional cerebral perfusion and carbondioxide reactivity: correlation with cerebral collaterals in internal carotid artery occlusive disease. J Neurol 253(10):1285–1291
Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, Carroll BA, Eliasziw M, Gocke J, Hertzberg BS, Katanick S, Needleman L, Pellerito J, Polak JF, Rholl KS, Wooster DL, Zierler RE (2003) Carotid artery stenosis: gray-scale and doppler US diagnosis-society of radiologists in ultrasound consensus conference. Radiology 229(2):340–346
Gunnal SA, Farooqui MS, Wabale RN (2014) Anatomical variations of the circulus arteriosus in cadaveric human brains. Neurol Res Int 687281
Henderson RD, Eliasziw M, Fox AJ, Rothwell PM, Barnett HJ (2000) Angiographically defined collateral circulation and risk of stroke in patients with severe carotid artery stenosis. Stroke 31(1):128–132
Husain I, Langdon C, Schwark J (2014) Three dimensional pulsatile non-Newtonian flow in a stenotic vessel. In: Computational problems in engineering. Springer International Publishing, pp 55–64
Jaff MR, Goldmakher GV, Lev MH, Romero JM (2008) Imaging of the carotid arteries: the role of duplex ultrasonography, magnetic resonance arteriography, and computerized tomographic arteriography. Vasc Med 13(4):281–292
Jozwik K, Obidowski D (2010) Numerical simulations of the blood flow through vertebral arteries. J Biomech 43(2):177–185
Kim HJ, Vignon-Clementel IE, Coogan JS, Figueroa CA, Jansen KE, Taylor CA (2010) Patient-specific modeling of blood flow and pressure in human coronary arteries. Ann Biomed Eng 38(10):3195–3209
Klein I, Lavallee P, Touboul P, Schouman-Claeys E, Amarenco P (2006) In vivo middle cerebral artery plaque imaging by high-resolution MRI. Neurology 67(2):327–329
Konala BC, Das A, Banerjee RK (2011) Influence of arterial wall-stenosis compliance on the coronary diagnostic parameters. J Biomech 44(5):842–847
Les AS, Shadden SC, Figueroa CA, Park JM, Tedesco MM, Herfkens RJ, Dalman RL, Taylor CA (2010) Quantification of hemodynamics in abdominal aortic aneurysms during rest and exercise using magnetic resonance imaging and computational fluid dynamics. Ann Biomed Eng 38(4):1288–1313
Lin W, Ma X, Deng D, Li Y (2015) Hemodynamics in the circle of Willis with internal carotid artery stenosis under cervical rotatory manipulation: a finite element analysis. Med Sci Monit 21:1820–1826
Lippert H, Pabst R (1985) Arterial variations in man: classification and frequency. Springer, Berlin
Long Q, Luppi L, König CS, Rinaldo V, Das SK (2008) Study of the collateral capacity of the circle of Willis of patients with severe carotid artery stenosis by 3D computational modeling’. J Biomech 41(12):2735–2742
MacDonald ME, Frayne R (2015) Phase contrast MR imaging measurements of blood flow in healthy human cerebral vessel segments. Physiol Meas 36(7):1517–1527
Markus HS, van der Worp HB, Rothwell PM (2013) Posterior circulation ischaemic stroke and transient ischaemic attack: diagnosis, investigation, and secondary prevention. Lancet Neurol 12(10):989–998
Moore S, David T, Chase JG, Arnold J, Fink J (2006) 3D models of blood flow in the cerebral vasculature. J Biomech 39(8):1454–1463
Moorhead KT, Doran CV, Chase JG, David T (2004) Lumped parameter and feedback control models of the auto-regulatory response in the circle of Willis. Comput Methods Biomech Biomed Engin 7(3):121–130
Oktar S, Yücel C, Karaosmanoglu D, Akkan K, Ozdemir H, Tokgoz N, Tali T (2006) Blood-flow volume quantification in internal carotid and vertebral arteries: comparison of 3 different ultrasound techniques with phase-contrast MR imaging. Am J Neuroradiol 27(2):363–369
Perren F, Poglia D, Landis T, Sztajzel R (2007) Vertebral artery hypoplasia A predisposing factor for posterior circulation stroke? Neurology 68(1):65–67
Purves D, George JA, David F, William CH, Anthony-Samuel L, James OM, Leonard EW (2008) Neuroscience, 4th edn. Sinauer Associates, Sunderland
Qiu C, Zhang Y, Xue C, Jiang S, Zhang W (2015) MRA study on variation of the circle of willis in healthy Chinese male adults. Biomed Res Int 976340
Reorowicz P, Obidowski D, Klosinski P, Szubert W, Stefanczyk L, Jozwik K (2014) Numerical simulations of the blood flow in the patient-specific arterial cerebral circle region. J Biomech 47(7):1642–1651
Reymond P, Perren F, Lazeyras F, Stergiopulos N (2012) Patient-specific mean pressure drop in the systemic arterial tree, a comparison between 1-D and 3-D models. J Biomech 45(15):2499–2505
Schmidt RF, Lang F, Heckmann M (2010) Physiologie des Menschen mit Pathophysiologie. Springer, Berlin
Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL (2000) MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31(7):1672–1678
Stergiopulos N, Young DF, Rogge TR (1992) Computer simulation of arterial flow with applications to arterial and aortic stenosis. J Biomech 25(12):1477–1488
Troianowski G, Taylor CA, Feinstein JA, Vignon-Clementel IE (2011) Three-dimensional simulations in Glenn patients: clinically based boundary conditions, hemodynamic results and sensitivity to input data. J Biomech Eng 133(11):111006
Tu S, Barbato E, Köszegi Z, Yang J, Sun Z, Holm NR, Tar B, Li Y, Rusinaru D, Wijns W, Reiber JHC (2014) Fractional flow reserve calculation from 3-dimensional quantitative coronary angiography and TIMI frame count: a fast computer model to quantify the functional significance of moderately obstructed coronary arteries. JACC Cardiovasc Interv 7(7):768–777
van Laar PJ, van der Grond J, Bremmer JP, Klijn CJ, Hendrikse J (2008) Assessment of the contribution of the external carotid artery to brain perfusion in patients with internal carotid artery occlusion. Stroke 39(11):3003–3008
Vignon-Clementel IE, Figueroa CA, Jansen KE, Taylor CA (2010) Outflow boundary conditions for 3D simulations of non-periodic blood flow and pressure fields in deformable arteries. Comput Methods Biomech Biomed Engin 13:625–640
Wellnhofer E, Osman J, Kertzscher U, Affeld K, Fleck E, Goubergrits L (2010) Flow simulation studies in coronary arteries-impact of side-branches. Atherosclerosis 213(2):475–481
Xiao N, Alastruey J, Figueroa CA (2014) A systematic comparison between 1-D and 3-D hemodynamics in compliant arterial models. Int J Numer Method Biomed Eng 30(2):204–231
Xie X, Wang Y, Zhu H, Zhou J (2014) Computation of hemodynamics in tortuous left coronary artery: a morphological parametric study. J Biomech Eng 136(10):101006
Zierler RE, Beach KW, Bergelin RO, Lal BK, Moore WS, Roubin GS, Voeks JH, Brott TG (2014) Agreement between site-reported and ultrasound core laboratory results for duplex ultrasound velocity measurements in the Carotid Revascularization Endarterectomy versus Stenting Trial. J Vasc Surg 59(1):2–7
Zuleger DI, Poulikakos D, Valavanis A, Kollias SS (2010) Combining magnetic resonance measurements with numerical simulations—extracting blood flow physiology information relevant to the investigation of intracranial aneurysms in the circle of Willis. Int J Heat Fluid Flow 31(6):1032–1039
Acknowledgments
This work was supported in part by Guangdong Image-guided Therapy Innovation Team (2011S013), the science technology, Shenzhen innovation funding of Government (CXZZ20140909004122087, SGLH20150213143207911, SGLH20150216172854731) and Innovation committee of Shenzhen (JCYJ20140414170821190, JCYJ20140901003939025, JCYJ20140414170821323, JCYJ20151030151431727).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, X., Gao, Z., Xiong, H. et al. Three-dimensional hemodynamics analysis of the circle of Willis in the patient-specific nonintegral arterial structures. Biomech Model Mechanobiol 15, 1439–1456 (2016). https://doi.org/10.1007/s10237-016-0773-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-016-0773-6