Abstract
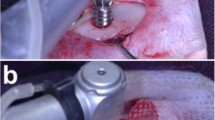
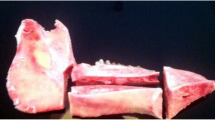
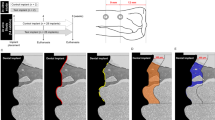
Because of its high predictability of success, implant therapy is a reliable treatment for replacement of missing teeth. The concept of immediate implant loading has been widely accepted in terms of early esthetic and functional recovery. However, there is little biological evidence to support this concept. The objective of this study was to examine the interactive effects of mechanical loading and surface roughness of immediately loaded titanium implants on bone formation in rats. Screw-shaped anodized titanium implants were either untreated (smooth) or acid-etched. Two implants were inserted parallel to each other in the tibiae of rats, and a closed coil spring (2.0 N) was immediately applied. Trabecular and cortical bone around both implants was analyzed using microtomographic images, and a removal torque test was performed at weeks 1, 2, and 4. Immediate loading of acid-etched implants resulted in significant decreases in bone mineral density, contact surface area, and cortical bone thickness. These effects were not observed after immediate loading of smooth implants. Conversely, loading did not influence acid-etched implant fixation; however, smooth implant fixation at week 1 was significantly reduced. These results imply that surface roughness regulates bone response to mechanical stress and that immediate loading might not inhibit osseointegration for smooth and rough implants in the late healing stages.








Similar content being viewed by others
References
Brånemark PI, Zarb G, Albrektsson T. Tissue-integrated prostheses: osseointegration in clinical dentistry. Chicago: Quintessence 1985:11–77.
Brånemark PI, Hansson B, Adell R, Breine U, Lindström J, Hallén O, Ohman A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl. 1977;16:1–132.
Boyan BD, Lossdorfer S, Wang L, Zhao G, Lohmann CH, Cochran DL, Schwartz Z. Osteoblasts generate an osteogenic microenvironment when grown on surfaces with rough microtopographies. Eur Cell Mater. 2003;6:22–7.
Schwartz Z, Lohmann CH, Vocke AK, Sylvia VL, Cochran DL, Dean DD, Boyan BD. Osteoblast response to titanium surface roughness and 1alpha, 25-(OH)(2)D(3) is mediated through the mitogen-activated protein kinase (MAPK) pathway. J Biomed Mater Res. 2001;56:417–26.
Takeuchi K, Saruwatari L, Nakamura HK, Yang JM, Ogawa T. Enhanced intrinsic biomechanical properties of osteoblastic mineralized tissue on roughened titanium surface. J Biomed Mater Res A. 2005;72:296–305.
Lai HC, Zhuang LF, Zhang ZY, Wieland M, Liu X. Bone apposition around two different sandblasted, large-grit and acid-etched implant surfaces at sites with coronal circumferential defects: an experimental study in dogs. Clin Oral Implants Res. 2009;20:247–53.
Butz F, Ogawa T, Nishimura I. Interfacial shear strength of endosseous implants. Int J Oral Maxillofac Implants. 2011;26:746–51.
Cooper LF. A role for surface topography in creating and maintaining bone at titanium endosseous implants. J Prosthet Dent. 2000;84:522–34.
Ogawa T, Nishimura I. Different bone integration profiles of turned and acid-etched implants associated with modulated expression of extracellular matrix genes. Int J Oral Maxillofac Implants. 2003;18:200–10.
Ogawa T, Ozawa S, Shih JH, Ryu KH, Sukotjo C, Yang JM, Nishimura I. Biomechanical evaluation of osseous implants having different surface topographies in rats. J Dent Res. 2000;79:1857–63.
Sanuki R, Shionome C, Kuwabara A, Mitsui N, Koyama Y, Suzuki N, Zhang F, Shimizu N, Maeno M. Compressive force induces osteoclast differentiation via prostaglandin E(2) production in MC3T3-E1 cells. Connect Tissue Res. 2010;51:150–8.
Balcells M, Fernandez Suarez M, Vazquez M, Edelman ER. Cells in fluidic environments are sensitive to flow frequency. J Cell Physiol. 2005;204:329–35.
Zhang X, Naert I, Van Schoonhoven D, Duyck J. Direct high-frequency stimulation of peri-implant rabbit bone: a pilot study. Clin Implant Dent Relat Res. 2010. doi:10.1111/j.1708-8208.2010.00298x.
Ogawa T, Possemiers T, Zhang X, Naert I, Chaudhari A, Sasaki K, Duyck J. Influence of whole-body vibration time on peri-implant bone healing: a histomorphometrical animal study. J Clin Periodontol. 2011;38:180–5.
Bannister SR, Lohmann CH, Liu Y, Sylvia VL, Cochran DL, Dean DD, Boyan BD, Schwartz Z. Shear force modulates osteoblast response to surface roughness. J Biomed Mater Res. 2002;60:167–74.
Frost HM. A determinant of bone architecture. The minimum effective strain. Clin Orthop Relat Res. 1983:286–92.
Hoshaw SJ, Brunski BJ, Cochran GVB. Mechanical loading of Brånemark implants affects interfacial bone modeling and remodeling. Int J Oral Maxillofac Implants. 1994;9:345–60.
Vandamme K, Naert I, Geris L, Vander Sloten J, Puers R, Duyck J. The effect of micro-motion on the tissue response around immediately loaded roughened titanium implants in the rabbit. Eur J Oral Sci. 2007;115:21–9.
De Smet E, Jaecques SV, Wevers M, Jansen JA, Jacobs R, Sloten JV, Naert IE. Effect of controlled early implant loading on bone healing and bone mass in guinea pigs, as assessed by micro-CT and histology. Eur J Oral Sci. 2006;114:232–42.
Isidor F. Histological evaluation of peri-implant bone at implants subjected to occlusal overload or plaque accumulation. Clin Oral Implants Res. 1997;8:1–9.
Isidor F. Mobility assessment with the periotest system in relation to histologic findings of oral implants. Int J Oral Maxillofac Implants. 1998;13:377–83.
Simmons CA, Meguid SA, Pilliar RM. Differences in osseointegration rate due to implant surface geometry can be explained by local tissue strains. J Orthop Res. 2001;19:187–94.
Wiskott HW, Belser UC. Lack of integration of smooth titanium surfaces: a working hypothesis based on strains generated in the surrounding bone. Clin Oral Implants Res. 1999;10:429–44.
Boyan BD, Sylvia VL, Liu Y, Sagun R, Cochran DL, Lohmann CH, Dean DD, Schwartz Z. Surface roughness mediates its effects on osteoblasts via protein kinase A and phospholipase A2. Biomaterials. 1999;20:2305–10.
Das K, Bose S, Bandyopadhyay A. TiO2 nanotubes on Ti: influence of nanoscale morphology on bone cell-materials interaction. J Biomed Mater Res A. 2009;90:225–37.
Vandamme K, Naert I, Vander Sloten J, Puers R, Duyck J. Effect of implant surface roughness and loading on peri-implant bone formation. Eur J Oral Sci. 2008;79:150–7.
Kawahara H, Aoki H, Koike H, Soeda Y, Kawahara D, Matsuda S. No evidence to indicate topographic dependency on bone formation around cp titanium implants under masticatory loading. J Mater Sci Mater Med. 2006;17:727–34.
Schneider GB, Zaharias R, Seabold D, Keller J, Stanford C. Differentiation of preosteoblasts is affected by implant surface microtopographies. J Biomed Mater Res A. 2004;69:462–8.
Turner CH. Three rules for bone adaptation to mechanical stimuli. Bone. 1998;23:399–407.
Sato N, Kubo K, Yamada M, Hori N, Suzuki T, Maeda H, Ogawa T. Osteoblast mechanoresponses on Ti with different surface topographies. J Dent Res. 2009;88:812–6.
Ingber DE, Tensegrity II. How structural networks influence cellular information processing networks. J Cell Sci. 2003;116:1397–408.
Pavalko FM, Norvell SM, Burr DB, Turner CH, Duncan RL, Bidwell JP. A model for mechanotransduction in bone cells: the load-bearing mechanosomes. J Cell Biochem. 2003;88:104–12.
Weinbaum S, Guo P, You L. A new view of mechanotransduction and strain amplification in cells with microvilli and cell processes. Biorheology. 2001;38:119–42.
Lange R, Luthen F, Beck U, Rychly J, Baumann A, Nebe B. Cell-extracellular matrix interaction and physico-chemical characteristics of titanium surfaces depend on the roughness of the material. Biomol Eng. 2002;19:255–61.
Anselme K, Bigerelle M, Noel B, Dufresne E, Judas D, Iost A, Hardouin P. Qualitative and quantitative study of human osteoblast adhesion on materials with various surface roughnesses. J Biomed Mater Res. 2000;49:155–66.
Sela MN, Badihi L, Rosen G, Steinberg D, Kohavi D. Adsorption of human plasma proteins to modified titanium surfaces. Clin Oral Implants Res. 2007;18:630–8.
Lanyon LE. Control of bone architecture by functional load bearing. J Bone Miner Res. 1992;7(Suppl 2):S369–75.
Medard C, Ribaux C, Chavrier C. A histological investigation on early tissue response to titanium implants in a rat intramedullary model. J Oral Implantol. 2000;26:238–43.
Guglielmotti MB, Renou S, Cabrini RL. Evaluation of bone tissue on metallic implants by energy-dispersive X-ray analysis: an experimental study. Implant Dent. 1999;8:303–9.
Kajiwara H, Yamaza T, Yoshinari M, Goto T, Iyama S, Atsuta I, Kido MA, Tanaka T. The bisphosphonate pamidronate on the surface of titanium stimulates bone formation around tibial implants in rats. Biomaterials. 2005;26:581–7.
Clokie CM, Warshawsky H. Morphologic and radioautographic studies of bone formation in relation to titanium implants using the rat tibia as a model. Int J Oral Maxillofac Implants. 1995;10:155–65.
Clokie CM, Warshawsky H. Development of a rat tibia model for morphological studies of the interface between bone and a titanium implant. Compendium 1995;16:56, 58, 60 passim (quiz 8).
Duyck J, Cooman MD, Puers R, Van Oosterwyck H, Sloten JV, Naert I. A repeated sampling bone chamber methodology for the evaluation of tissue differentiation and bone adaptation around titanium implants under controlled mechanical conditions. J Biomech. 2004;37:1819–22.
Duyck J, Slaets E, Sasaguri K, Vandamme K, Naert I. Effect of intermittent loading and surface roughness on peri-implant bone formation in a bone chamber model. J Clin Periodontol. 2007;34:998–1006.
Melsen B, Lang NP. Biological reactions of alveolar bone to orthodontic loading of oral implants. Clin Oral Implants Res. 2001;12:144–52.
Gotfredsen K, Berglundh T, Lindhe J. Bone reactions adjacent to titanium implants subjected to static load. A study in the dog (I). Clin Oral Implants Res. 2001;12:1–8.
Gotfredsen K, Berglundh T, Lindhe J. Bone reactions adjacent to titanium implants with different surface characteristics subjected to static load. A study in the dog (II). Clin Oral Implants Res. 2001;12:196–201.
Gotfredsen K, Berglundh T, Lindhe J. Bone reactions adjacent to titanium implants subjected to static load of different duration. A study in the dog (III). Clin Oral Implants Res. 2001;12:552–8.
Shigemitsu R, Ogawa T, Matsumoto T, Yoda N, Gunji Y, Yamakawa Y, Ikeda K, Sasaki K. Stress distribution in the peri-implant bone with splinted and non-splinted implants by in vivo loading data-based finite element analysis. Odontology. 2012. doi:10.1007/s10266-012-0077-y.
Chatzigianni A, Keilig L, Duschner H, Götz H, Eliades T, Bourauel C. Comparative analysis of numerical and experimental data of orthodontic mini-implants. Eur J Orthod. 2011;33:468–75.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (grant no. 21791876) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflict of Interest
None of the authors have any conflicts of interest associated with this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sato, N., Kuwana, T., Yamamoto, M. et al. Bone response to immediate loading through titanium implants with different surface roughness in rats. Odontology 102, 249–258 (2014). https://doi.org/10.1007/s10266-013-0107-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-013-0107-4