Abstract
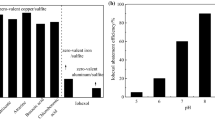
There is actually a need for efficient methods to clean waters and wastewaters from pollutants such as the bisphenol A endocrine disrupter. Advanced oxidation processes currently use persulfate or peroxymonosulfate to generate sulfate radicals. There are, however, few reports on the use of sulfite to generate sulfate radicals, instead of persulfate or peroxymonosulfate, except for dyes. Here we studied the degradation of the bisphenol A using iron(III) as catalyst and sulfite as precursor of oxysulfur radicals, at initial pH of 6, under UV irradiation at 395 nm. The occurrence of radicals was checked by quenching with tert-butyl alcohol and ethanol. Bisphenol A degradation products were analyzed by liquid chromatography coupled with mass spectrometry (LC–MS). Results reveal that iron(III) or iron(II) have a similar oxidation efficiency. Quenching experiments show that the oxidation rate of bisphenol A is 47.7 % for SO ·−4 , 37.3 % for SO ·−5 and 15 % for HO·. Bisphenol A degradation products include catechol and quinone derivatives. Overall, our findings show that the photo-iron(III)–sulfite system is efficient for the oxidation of bisphenol A at circumneutral pH.



Similar content being viewed by others
References
Anipsitakis GP, Dionysiou DD (2004) Radical generation by the interaction of transition metals with common oxidants. Environ Sci Technol 38:3705–3712. doi:10.1021/es035121o
Brandt C, Fabian I, van Eldik R (1999) Kinetics and mechanism of the iron(III)-catalyzed autoxidation of sulfur(IV) oxides in aqueous solution: evidence for the redox cycling of iron in the presence of oxygen and modeling of the overall reaction mechanism. Inorg Chem 33:687–701. doi:10.1021/ic00082a012
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O−) in aqueous solution. J Phys Chem Ref Data 17:513–886. doi:10.1063/1.555805
Chen L, Peng X, Liu J, Li J, Wu F (2012) Decolorization of orange II in aqueous solution by an Fe(II)/sulfite system: replacement of persulfate. Ind Eng Chem Res 51:13632–13638. doi:10.1021/ie3020389
Das NT, Huie ER, Neta P (1999) Reduction potentials of SO3 −, SO5 − and S4O6 3− radicals in aqueous solution. J Phys Chem A 103:3581–3588. doi:10.1021/jp9900234
Hammedi T, Triki M, Alvarez MG, Chimentao RJ, Ksibi Z, Ghorbel A, Llorca J, Medina F (2015) Total degradation of p-hydroxybenzoic acid by Ru-catalysed wet air oxidation: a model for wastewater treatment. Environ Chem Lett 13:481–486. doi:10.1007/s10311-015-0529-z
Hayon E, Treinin A, Wilf J (1972) Electronic spectra, photochemistry, and autoxidation mechanism of the sulfite–bisulfite–pyrosulfite systems. J Am Chem Soc 94:47–57. doi:10.1021/ja00756a009
Huang W, Brigante M, Wu F, Mousty C, Hanna K, Mailhot G (2013) Assessment of the Fe(III)–EDDS complex in Fenton-like processes: from the radical formation to the degradation of bisphenol A. Environ Sci Technol 47:1952–1959. doi:10.1021/es304502y
Jiang X, Wu Y, Wang P, Li H, Dong W (2013) Degradation of bisphenol A in aqueous solution by persulfate activated with ferrous ion. Environ Sci Pollut Res 20:4947–4953. doi:10.1007/s11356-013-1468-5
Lee HJ, Kim HH, Lee H, Lee C (2015) Reaction of aqueous iodide at high concentration with O3 and O3/H2O2 in the presence of natural organic matter: implications for drinking water treatment. Environ Chem Lett 13:453–458. doi:10.1007/s10311-015-0519-1
Liang C, Su HW (2009) Identification of sulfate and hydroxyl radicals in thermally activated persulfate. Ind Eng Chem Res 48:5558–5562. doi:10.1021/ie9002848
McLachlan GA, Muller JG, Rokita SE, Burrows CJ (1996) Metal-mediated oxidation of guanines in DNA and RNA: a comparison of cobalt(II), nickel(II) and copper(II) complexes. Inorg Chim Acta 251:193–199. doi:10.1016/s0020-1693(96)05272-3
Minero C, Maurino V, Pelizzetti E, Vione D (2006) An empirical, quantitative approach to predict the reactivity of some substituted aromatic compounds towards reactive radical species (Cl −·2 , Br −·2 , ·NO2, SO −·3 , SO −·4 ) in aqueous solution. Environ Sci Pollut Res 13:212–214. doi:10.1065/espr2006.06.318
Neta P, Huie RE (1985) One-electron redox reactions involving sulfite ions and aromatic amines. J Phys Chem 89:1783–1787. doi:10.1021/j100255a049
Neta P, Huie RE, Ross AB (1988) Rate constants for reactions of inorganic radicals in aqueous solution. J Phys Chem Ref Data 17:1027–1284. doi:10.1063/1.555808
Rodriguez EM, Fernandez G, Klamerth N, Maldonado MI, Alvarez PM, Malato S (2010) Efficiency of different solar advanced oxidation processes on the oxidation of bisphenol A in water. Appl Catal B Environ 95:228–237. doi:10.1016/j.apcatb.2009.12.027
Sajiki J, Yonekubo J (2003) Leaching of bisphenol A (BPA) to seawater from polycarbonate plastic and its degradation by reactive oxygen species. Chemosphere 51:55–62. doi:10.1016/s0045-6535(02)00789-0
Seinfeld JH, Pandis SN (1998) Atmospheric chemistry and physics: from air pollution to climate change. Wiley, New York
Shukla S, Oturan MA (2015) Dye removal using electrochemistry and semiconductor oxide nanotubes. Environ Chem Lett 13:157–172. doi:10.1007/s10311-015-0501-y
Wine PH, Tang Y, Thorn RP, Wells JR, Davis DD (1989) Kinetics of aqueous phase reactions of the SO −·4 radical with potential importance in cloud chemistry. J Geophys Res 94:1085–1094. doi:10.1029/jd094id01p01085
Yates BJ, Darlington R, Zboril R, Sharma VK (2014) High-valent iron-based oxidants to treat perfluorooctanesulfonate and perfluorooctanoic acid in water. Environ Chem Lett 12:413–417. doi:10.1007/s10311-014-0463-5
Zhang W, Singh P, Muir D (2000) Iron(II) oxidation by SO2/O2 in acidic media: Part I. Kinetics and mechanism. Hydrometallurgy 55:229–245. doi:10.1016/s0304-386x(99)00082-1
Zhang L, Chen L, Xiao M, Zhang L, Wu F, Ge L (2013) Enhanced decolorization of orange II solutions by the Fe(II)–sulfite system under xenon lamp irradiation. Ind Eng Chem Res 52:10089–10094. doi:10.1021/ie400469u
Zhou D, Wu F, Deng N, Xiang W (2004) Photooxidation of bisphenol A (BPA) in water in the presence of ferric and carboxylate salts. Water Res 38:4107–4116. doi:10.1016/j.watres.2004.07.021
Zhou D, Chen L, Zhang C, Yu Y, Zhang L, Wu F (2014a) A novel photochemical system of ferrous sulfite complex: kinetics and mechanisms of rapid decolorization of acid orange 7 in aqueous solutions. Water Res 57:87–95. doi:10.1016/j.watres.2014.03.016
Zhou D, Zhang H, Chen L (2014b) Sulfur-replaced Fenton systems: can sulfate radical substitute hydroxyl radical for advanced oxidation technologies? J Chem Technol Biotechnol 90:775–779. doi:10.1002/jctb.4525
Zhou D, Yuan Y, Yang S, Gao H, Chen L (2015) Roles of oxysulfur radicals in the oxidation of acid orange 7 in the Fe(III)–sulfite system. J Sulfur Chem 36:373–384. doi:10.1080/17415993.2015.1028939
Zhu Z, Zuo Y (2013) Bisphenol A and other alkylphenols in the environment—occurrence, fate, health effects and analytical techniques. Adv Environ Res 2:179–202. doi:10.12989/aer.2013.2.3.179
Acknowledgments
This work was supported by the Natural Science Foundation of China (No. 21477090). Comments from anonymous reviewers are also appreciated.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yu, Y., Li, S., Peng, X. et al. Efficient oxidation of bisphenol A with oxysulfur radicals generated by iron-catalyzed autoxidation of sulfite at circumneutral pH under UV irradiation. Environ Chem Lett 14, 527–532 (2016). https://doi.org/10.1007/s10311-016-0573-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-016-0573-3