Abstract
Object
The aim of our study was to enable automatic volumetry of the entire kidneys as well as their internal structures (cortex, medulla, and pelvis) from native magnetic resonance imaging (MRI) data sets.
Materials and methods
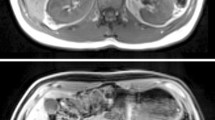
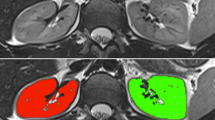
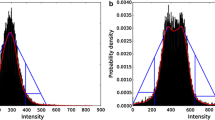
Segmentation of the entire kidneys and differentiation of their internal structures were performed in 12 healthy volunteers based on non-contrast-enhanced T1- and T2-weighted MR images. Two data sets (each acquired in one breath-hold) were co-registered using a rigid registration algorithm compensating for possible breathing-related displacements. An automatic algorithm based on thresholding and shape detection segmented the kidneys into their compartments and was compared to a manual labeling procedure.
Results
The resulting kidney volumes of the automated segmentation correlated well with those created manually (R 2 = 0.96). Average volume errors were determined to be 4.97 ± 4.08 % (entire kidney parenchyma), 7.03 ± 5.56 % (cortex), 12.33 ± 7.35 % (medulla), and 17.57 ± 14.47 % (pelvis). The variation of the kidney volume resulting from the automatic algorithm was found to be 4.76 % based on the measuring of one volunteer with three independent examinations.
Conclusion
The results demonstrate the feasibility of an accurate and repeatable automatic segmentation of the kidneys and their internal structures from non-contrast-enhanced magnetic resonance images.





Similar content being viewed by others
References
Jones RA, Easley K, Little SB, Scherz H, Kirsch AJ, Grattan-Smith JD (2005) Dynamic contrast-enhanced MR urography in the evaluation of pediatric hydronephrosis: Part I, functional assessment. AJR Am J Roentgenol 185(6):1598–1607
Karstoft K, Lodrup AB, Dissing TH, Sorensen TS, Nyengaard JR, Pederson M (2007) Different strategies for MRI measurements of renal cortical volume. J Magn Reson Imaging 26(6):1564–1571
Bakker J, Olree M, Kaatee R, de Lange EE, Moons KG, Beutler JJ, Beek FJ (1999) Renal volume measurements: accuracy and repeatability of US compared with that of MR imaging. Radiology 211(3):623–628
Bakker J, Olree M, Kaatee R, de Lange EE, Beek FJ (1998) In vitro measurement of kidney size: comparison of ultrasonography and MRI. Ultrasound Med Biol 24(5):683–688
Li S, Zoellner F, Merrem AD, Peng Y, Roervik J, Lundervold A, Schad LR (2012) Wavelet-based segmentation of renal compartments in DCE-MRI of human kidney: initial results in patients and healthy volunteers. Comput Med Imaging 36(2):108–118
Chevaillier B, Mandry D, Ponvianne Y, Collette Jl, Claudon M, Pietquin O (2008) Functional semi-automated segmentation of renal DCE-MRI sequences using a growing neural gas algorithm. In: 16th European Signal Processing Conference (EUSIPCO 2008), Lausanne
Grenier N, Basseau F, Ries M, Tyndal B, Jones R, Moonen C (2003) Functional MRI of the kidney. Abdom Imaging 28(2):164–175
Coulam CH, Bouley DM, Sommer FG (2002) Measurement of renal volumes with contrast-enhanced MRI. J Magn Reson Imaging 15(2):174–179
De Bazelaire CM, Duhamel GD, Rofsky NM, Alsop DC (2004) MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology 230(3):652–659
Di Leo G, Di Terlizzi F, Flor N, Morganti A, Sardanelli F (2011) Measurement of renal volume using respiratory-gated MRI in subjects without known kidney disease: intraobserver, interobserver, and interstudy reproducibility. Eur J Radiol 80(3):e212–e216
Kanki A, Ito K, Tamada T, Noda Y, Yamamoto A, Tanimoto D, Sato T, Higaki A (2013) Corticomedullary differentiation of the kidney: evaluation with noncontrast-enhanced steady-state free precession (SSFP) MRI with time-spatial labeling inversion pulse (time-SLIP). J Magn Reson Imaging 37(5):1178–1181
Cohen BA, Barash I, Kim DC, Sanger MD, Babb JS, Chandarana H (2012) Intraobserver and interobserver variability of renal volume measurements in polycystic kidney disease using a semiautomated MR segmentation algorithm. AJR Am J Roentgenol 199(2):387–393
Tang Y, Jackson HA, De Filippo RE, Nelson MD, Moats RA (2010) Automatic renal segmentation applied in pediatric MR urography. Int J Intell Inf Process 1(1):12–19
Borgelt C, Timm H, Kruse R (2000) Using fuzzy clustering to improve naive Bayes classifiers and probabilistic networks. In: Fuzzy Systems, The Ninth IEEE International Conference on, San Antonio, 1:53-58
Sun Y, Moura J, Yang D, Ye Q, Ho C (2002) Kidney segmentation in MRI sequences using temporal dynamics. IEEE Int Symp Biomed Imag 98–101
Vivier PH, Dolores M, Gardin I, Zhang P, Petitjean C, Dacher JN (2008) In vitro assessment of a 3D segmentation algorithm based on the belief functions theory in calculation renal volumes by MRI. AJR Am J Roentgenol 191(3):W127–W134
He L, Peng Z, Everding B, Wang X, Han CY, Weiss KL, Wee WG (2008) A comparative study of deformable contour methods on medical image segmentation. Image Vis Computing 26:141–163
Klein S, Staring M, Murphy K, Viergever MA, Pluim JP (2010) Elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging 29(1):196–205
Kass M, Witkin A, Terzopoulos D (1988) Snakes: active contour models. Int J Comput Vis 1:321–331
Zöllner FG, Svarstad E, Munthe-Kaas AZ, Schad LR, Lundervold A, Rorvik J (2012) Assessment of kidney volumes from MRI: acquisition and segmentation techniques. Am J Roentgenol 199(5):1060–1069
Cheong B, Muthupillai R, Rubin MF, Flamm SD (2007) Normal values for renal length and volume as measured by magnetic resonance imaging. Clin J Am Soc Nephrol 2(1):38–45
Gloger O, Tönies KD, Liebscher V, Kugelmann B, Laqua R, Völzke H (2012) Prior shape level set segmentation on multistep generated probability maps of MR datasets for fully automated kidney parenchyma volumetry. IEEE Trans Med Imaging 31(2):312–325
González Ballester MA, Zissermann AP, Brady M (2002) Estimation of the partial volume effect in MRI. Med Image Anal 6(4):389–405
Pham D, KronT, Foroudi F, Schneider M, Siva S (2013) A review of kidney motion under free, deep and forced-shallow breathing conditions: implications for stereotactic ablative body radiotherapy treatment. Technol Cancer Res Treat
Gadeberg P, Gundersen HJ, Taagehoj F (1999) How accurate are measurements on MRI? A study on multiple sclerosis using reliable 3D stereological methods. J Magn Reson Imaging 10(1):72–79
Lee VS, Kaur M, Bokacheva L, Chen Q, Rusinek H, Thakur R, Moses D, Nazzaro C, Kramer EL (2007) What causes diminished corticomedullary differentiation in renal insufficiency? J Magn Reson Imaging 25(4):790–795
Mounier-Vehier C, Lions C, Devos P, Jaboureck O, Willoteaux S, Carre A, Beregi JP (2002) Cortical thickness: an early morphological marker of atherosclerotic renal disease. Kidney Int 61(2):591–598
Roger SD, Beale AM, Cattell WR, Webb JA (1994) What is the value of measuring renal parenchymal thickness before renal biopsy? Clin Radiol 49(1):45–49
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Will, S., Martirosian, P., Würslin, C. et al. Automated segmentation and volumetric analysis of renal cortex, medulla, and pelvis based on non-contrast-enhanced T1- and T2-weighted MR images. Magn Reson Mater Phy 27, 445–454 (2014). https://doi.org/10.1007/s10334-014-0429-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-014-0429-4