Abstract
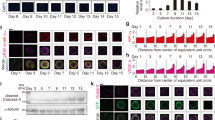
The formation of three-dimensional (3D) multicellular cell spheroids such as microspheres and embryoid bodies has recently gained much attention as a useful cell culture technique, but few studies have investigated the suitability of glass for spheroids formation and culture. In this work, we present a novel three-dimensional microfluidic device made of poly(dimethylsiloxane) (PDMS) and glass for the easy and rapid synthesis and culture of tumor spheroid. The cell culture unit is composed of an array of microwells on the bottom of a glass plate, bigger microwells and elastomeric microchannels on the top of a PDMS plate. Cell suspension can be easily introduced into the cell culture unit and exchange with the external liquid environment by the microfluidic channels. A single tumor spheroid can be formed and cultured in each glass cell culture chamber, the surface of which was modified with poly(vinyl alcohol) to render it to be resistant to cell adhesion. As the cell culture medium could be replaced, spheroids of the human breast cancer (MCF-7) cells were cultured on the chip for 3 days, reaching the diameters of about 150 μm. Furthermore, the MCF-7 cells were successfully cultured on the chip in 2D and 3D culture modes. Results have shown that glass is well suitable for multicellular tumor spheroids culture. The established platform provides a convenient and rapid method for tumor spheroid culture, which is also adaptable for anticancer drug screening and fundamental biomedical research in cell biology.






Similar content being viewed by others
References
Agastin S, Giang UBT, Geng Y, DeLouise LA, King MR (2011) Continuously perfused microbubble array for 3D tumor spheroid model. Biomicrofluidics 5(2). doi:10.1063/1.3596530
Birgersdotter A, Sandberg R, Ernberg I (2005) Gene expression perturbation in vitro—a growing case for three-dimensional (3D) culture systems. Semin Cancer Biol 15(5):405–412. doi:10.1016/j.semcancer.2005.06.009
Chen MCW, Gupta M, Cheung KC (2010a) Alginate-based microfluidic system for tumor spheroid formation and anticancer agent screening. Biomed Microdevices 12(4):647–654. doi:10.1007/s10544-010-9417-2
Chen Y, Choi JY, Choi SJ, Seo TS (2010b) Sample stacking capillary electrophoretic microdevice for highly sensitive mini Y short tandem repeat genotyping. Electrophoresis 31(17):2974–2980. doi:10.1002/elps.201000270
El-Ali J, Sorger PK, Jensen KF (2006) Cells on chips. Nature 442(7101):403–411. doi:10.1038/nature05063
Gao D, Liu JJ, Wei HB, Li HF, Guo GS, Lin JM (2010) A microfluidic approach for anticancer drug analysis based on hydrogel encapsulated tumor cells. Anal Chim Acta 665(1):7–14. doi:10.1016/j.aca.2010.03.015
Gao D, Liu HX, Jiang YY, Lin JM (2012) Recent developments in microfluidic devices for in vitro cell culture for cell-biology research. Trac-Trends Anal Chem 35:150–164. doi:10.1016/j.trac.2012.02.008
Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA (2010) Multicellular tumor spheroids: An underestimated tool is catching up again. J Biotechnol 148(1):3–15. doi:10.1016/j.jbiotec.2010.01.012
Hsiao AY, Torisawa YS, Tung YC, Sud S, Taichman RS, Pienta KJ, Takayama S (2009) Microfluidic system for formation of PC-3 prostate cancer co-culture spheroids. Biomaterials 30(16):3020–3027. doi:10.1016/j.biomaterials.2009.02.047
Huang P-H, Xie Y, Ahmed D, Rufo J, Nama N, Chen Y, Chan CY, Huang TJ (2013) An acoustofluidic micromixer based on oscillating sidewall sharp-edges. Lab Chip 13(19):3847–3852. doi:10.1039/C3LC50568E
Jedrych E, Pawlicka Z, Chudy M, Dybko A, Brzozka Z (2011) Evaluation of photodynamic therapy (PDT) procedures using microfluidic system. Anal Chim Acta 683(2):149–155. doi:10.1016/j.aca.2010.10.005
Jin H-J, Cho Y-H, Gu J-M, Kim J, Oh Y-S (2011) A multicellular spheroid formation and extraction chip using removable cell trapping barriers. Lab Chip 11(1):115–119. doi:10.1039/c0lc00134a
Kalchman J, Fujioka S, Chung S, Kikkawa Y, Mitaka T, Kamm RD, Tanishita K, Sudo R (2013) A three-dimensional microfluidic tumor cell migration assay to screen the effect of anti-migratory drugs and interstitial flow. Microfluid Nanofluid 14(6):969–981. doi:10.1007/s10404-012-1104-6
Karp JM, Yeh J, Eng G, Fukuda J, Blumling J, Suh K-Y, Cheng J, Mahdavi A, Borenstein J, Langer R, Khademhosseini A (2007) Controlling size, shape and homogeneity of embryoid bodies using poly(ethylene glycol) microwells. Lab Chip 7(6):786–794. doi:10.1039/b705085m
Kelm JM, Timmins NE, Brown CJ, Fussenegger M, Nielsen LK (2003) Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol Bioeng 83(2):173–180. doi:10.1002/bit.10655
Kim JB (2005) Three-dimensional tissue culture models in cancer biology. Semin Cancer Biol 15(5):365–377. doi:10.1016/j.semcancer.2005.05.002
Kim L, Toh Y-C, Voldman J, Yu H (2007) A practical guide to microfluidic perfusion culture of adherent mammalian cells. Lab Chip 7(6):681–694. doi:10.1039/b704602b
Kim C, Bang JH, Kim YE, Lee SH, Kang JY (2012a) On-chip anticancer drug test of regular tumor spheroids formed in microwells by a distributive microchannel network. Lab Chip 12(20):4135–4142. doi:10.1039/c21c40570a
Kim T, Doh I, Cho YH (2012b) On-chip three-dimensional tumor spheroid formation and pump-less perfusion culture using gravity-driven cell aggregation and balanced droplet dispensing. Biomicrofluidics 6(3). doi:10.1063/1.4739460
Liu WM, Sun P, Yang LY, Wang JF, Li L, Wang JY (2010) Assay of glioma cell responses to an anticancer drug in a cell-based microfluidic device. Microfluid Nanofluid 9(4–5):717–725. doi:10.1007/s10404-010-0584-5
Mori R, Sakai Y, Nakazawa K (2008) Micropatterned organoid culture of rat hepatocytes and HepG2 cells. J Biosci Bioeng 106(3):237–242. doi:10.1263/jbb.106.237
Nuttelman CR, Mortisen DJ, Henry SM, Anseth KS (2001) Attachment of fibronectin to poly(vinyl alcohol) hydrogels promotes NIH3T3 cell adhesion, proliferation, and migration. J Biomed Mater Res 57(2):217–223. doi:10.1002/1097-4636(200111)57:2<217:aid-jbm1161>3.0.co;2-i
Ozawa F, Ino K, Arai T, Ramon-Azcon J, Takahashi Y, Shiku H, Matsue T (2013) Alginate gel microwell arrays using electrodeposition for three-dimensional cell culture. Lab Chip. doi:10.1039/C3LC50455G
Sahai R, Cecchini M, Klingauf M, Ferrari A, Martino C, Castrataro P, Lionetti V, Menciassi A, Beltram F (2011) Microfluidic chip for spatially and temporally controlled biochemical gradient generation in standard cell-culture Petri dishes. Microfluid Nanofluid 11(6):763–771. doi:10.1007/s10404-011-0841-2
Sakai Y, Nakazawa K (2007) Technique for the control of spheroid diameter using microfabricated chips. Acta Biomater 3(6):1033–1040. doi:10.1016/j.actbio.2007.06.004
Sakai Y, Yoshida S, Yoshiura Y, Mori R, Tamura T, Yahiro K, Mori H, Kanemura Y, Yamasaki M, Nakazawa K (2010) Effect of microwell chip structure on cell microsphere production of various animal cells. J Biosci Bioeng 110(2):223–229. doi:10.1016/j.jbiosc.2010.01.021
Shi Y, Gao XH, Chen LQ, Zhang M, Ma JY, Zhang XX, Qin JH (2013) High throughput generation and trapping of individual agarose microgel using microfluidic approach. Microfluid Nanofluid 15(4):467–474. doi:10.1007/s10404-013-1160-6
Sun Y, Yin XF (2006) Novel multi-depth microfluidic chip for single cell analysis. J Chromatogr A 1117(2):228–233. doi:10.1016/j.chroma.2006.03.088
Torisawa Y-s, Takagi A, Nashimoto Y, Yasukawa T, Shiku H, Matsue T (2007) A multicellular spheroid array to and viability realize spheroid formation, culture, assay on a chip. Biomaterials 28(3):559–566. doi:10.1016/j.biomaterials.2006.08.054
Wlodkowic D, Faley S, Skommer J, McGuinness D, Cooper JM (2009) Biological implications of polymeric microdevices for live cell assays. Anal Chem 81(23):9828–9833. doi:10.1021/ac902010s
Wong SF, No DY, Choi YY, Kim DS, Chung BG, Lee SH (2011) Concave microwell based size-controllable hepatosphere as a three-dimensional liver tissue model. Biomaterials 32(32):8087–8096. doi:10.1016/j.biomaterials.2011.07.028
Wu LY, Di Carlo D, Lee LP (2008) Microfluidic self-assembly of tumor spheroids for anticancer drug discovery. Biomed Microdevices 10(2):197–202. doi:10.1007/s10544-007-9125-8
Xu BY, Yan XN, Zhang JD, Xu JJ, Chen HY (2012) Glass etching to bridge micro- and nanofluidics. Lab Chip 12(2):381–386. doi:10.1039/c1lc20741e
Xu BY, Hu SW, Qian GS, Xu JJ, Chen HY (2013) A novel microfluidic platform with stable concentration gradient for on chip cell culture and screening assays. Lab Chip 13(18):3714–3720. doi:10.1039/c3lc50676b
Yamada KM, Cukierman E (2007) Modeling tissue morphogenesis and cancer in 3D. Cell 130(4):601–610. doi:10.1016/j.cell.2007.08.006
Yang F, Chen ZG, Pan JB, Li XC, Feng J, Yang H (2011) An integrated microfluidic array system for evaluating toxicity and teratogenicity of drugs on embryonic zebrafish developmental dynamics. Biomicrofluidics 5(2). doi:10.1063/1.3605509
Yang J, Chen Z, Ching P, Shi Q, Li X (2013) An integrated microfluidic platform for evaluating in vivo antimicrobial activity of natural compounds using a whole-animal infection model. Lab Chip. doi:10.1039/C3LC50264C
Yoon S, Kim JA, Lee SH, Kim M, Park TH (2013) Droplet-based microfluidic system to form and separate multicellular spheroids using magnetic nanoparticles. Lab Chip 13(8):1522–1528. doi:10.1039/c3lc41322e
Yu LF, Chen MCW, Cheung KC (2010) Droplet-based microfluidic system for multicellular tumor spheroid formation and anticancer drug testing. Lab Chip 10(18):2424–2432. doi:10.1039/c004590j
Zajaczkowski MB, Cukierman E, Galbraith CG, Yamada KM (2003) Cell-matrix adhesions on poly(vinyl alcohol) hydrogels. Tissue Eng 9(3):525–533. doi:10.1089/107632703322066705
Ziolkowska K, Kwapiszewski R, Stelmachowska A, Chudy M, Dybko A, Brzozka Z (2012) Development of a three-dimensional microfluidic system for long-term tumor spheroid culture. Sens Actuators B-Chem 173:908–913. doi:10.1016/j.snb.2012.07.045
Ziolkowska K, Stelmachowska A, Kwapiszewski R, Chudy M, Dybko A, Brzozka Z (2013) Long-term three-dimensional cell culture and anticancer drug activity evaluation in a microfluidic chip. Biosens Bioelectron 40(1):68–74. doi:10.1016/j.bios.2012.06.017
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 21375152 and 21075139). The authors thank Dr. Karina Ziolkowska (Warsaw University of Technology, Department of Microbioanalytics, Warsaw, Poland), Dr. Dan Gao (The Key Laboratory of Chemical Biology, Tsinghua University, Shenzhen, China) for valuable discussion and Prof. Peiqing Liu (New Drug Screening Center, Sun Yat-sen University) for the help of cell culture.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, D., Lu, J., Chen, Z. et al. A novel three-dimensional microfluidic platform for on chip multicellular tumor spheroid formation and culture. Microfluid Nanofluid 17, 831–842 (2014). https://doi.org/10.1007/s10404-014-1373-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-014-1373-3