Abstract
The micro-separator constructed with periodic bottom microelectrodes and a continuous top electrode has been developed previously for continuous cell sorting by dielectrophoresis. To understand its full potential and to facilitate future device development, the working mechanism of such electrode design is investigated by mathematical simulation. We first present a unit-cell methodology to model spatial electric field strength (E) over the microelectrode space due to its periodic nature. Unit-cell methodology is useful in modeling of E distribution for the microelectrode space, allowing computing spatial dielectrophoretic force with much less computational efforts. By using the computed dielectrophoretic force, a cell motion model, which takes into consideration the dielectrophoretic, Stokes, buoyancy and gravitational forces on a cell, is established for the prediction of cell trajectory over the separation channel. This study demonstrates the validity of this model in predicting live and dead NIH-3T3 cells motions over the microelectrode space of the micro-separator by comparing numerical and experimental results. In conclusion, a mathematical model that combines unit-cell methodology and cell motion model has been proposed and has demonstrated its potential use as an effective tool for the evaluation of electrode with a periodic nature.





Similar content being viewed by others
References
Alazzam A, Roman D, Nerguizian V, Stiharu I, Bhat R (2010) Analytical formulation of electric field and dielectrophoretic force for moving dielectrophoresis using Fourier series. Microfluid Nanofluid 9:1115–1124
Beech JP, Jonsson P, Tegenfeldt JO (2009) Tipping the balance of deterministic lateral displacement devices using dielectrophoresis. Lab Chip 9:2698–2706
Chang S, Cho YH (2008) A continuous size-dependent particle separator using a negative dielectrophoretic virtual pillar array. Lab Chip 8:1930–1936
Choi S, Park JK (2005) Microfluidic system for dielectrophoretic separation based on a trapezoidal electrode array. Lab Chip 5:1161–1167
Ciprian I, Guo Lin X, Victor S, Francis EHT (2005) Fabrication of a dielectrophoretic chip with 3D silicon electrodes. J Micromech Microeng 15:494
Clague DS, Wheeler EK (2001) Dielectrophoretic manipulation of macromolecules: the electric field. Phys Rev E 64:026605
Deen WM (1998) Analysis of transport phenomena. Oxford University Press, New York
Demierre N, Braschler T, Muller R, Renaud P (2008) Focusing and continuous separation of cells in a microfluidic device using lateral dielectrophoresis. Sens Actuators, B 132:388–396
Fuhr G, Glasser H, Muller T, Schnelle T (1994) Cell manipulation and cultivation under ac electric field influence in highly conductive culture media. Biochim Biophys Acta Gen Subj 1201:353–360
Ganatos P (1978) A numerical solution technique for three-dimensional multiparticle stokes flow. Dissertation, The City University of New York
Ganatos P, Pfeffer R, Weinbaum S (1980a) A strong interaction theory for the creeping motion of a sphere between plane parallel boundaries. Part 2. Parallel motion. J Fluid Mech 99:755–783
Ganatos P, Weinbaum S, Pfeffer R (1980b) A strong interaction theory for the creeping motion of a sphere between plane parallel boundaries. Part 1. Perpendicular motion. J Fluid Mech 99:739–753
Gupta V, Jafferji I, Garza M, Melnikova VO, Hasegawa DK, Pethig R, Davis DW (2012) ApoStream™, a new dielectrophoretic device for antibody independent isolation and recovery of viable cancer cells from blood. Biomicrofluidics 6:024133-024114
Han KH, Han SI, Frazier AB (2009) Lateral displacement as a function of particle size using a piecewise curved planar interdigitated electrode array. Lab Chip 9:2958–2964
Hughes MP (2002) Strategies for dielectrophoretic separation in laboratory-on-a-chip systems. Electrophoresis 23:2569–2582
Jones TB (1995) Electromechanics of particles. Cambridge University Press, New York
Kang Y, Li D (2009) Electrokinetic motion of particles and cells in microchannels. Microfluid Nanofluid 6:431–460
Kang JH, Park JK (2005) Technical paper on microfluidic devices—cell separation technology. Asia Pacific Biotech News 9:1135–1146
Kim U, Shu CW, Dane KY, Daugherty PS, Wang JYJ, Soh HT (2007) Selection of mammalian cells based on their cell-cycle phase using dielectrophoresis. Proc Natl Acad Sci USA 104:20708–20712
Kim U, Qian J, Kenrick SA, Daugherty PS, Soh HT (2008) Multitarget dielectrophoresis activated cell sorter. Anal Chem 80:8656–8661
Kua CH, Lam YC, Rodriguez I, Yang C, Youcef-Toumi K (2008a) Cell motion model for moving dielectrophoresis. Anal Chem 80:5454–5461
Kua CH, Lam YC, Yang C, Youcef-Toumi K, Rodriguez I (2008b) Modeling of dielectrophoretic force for moving dielectrophoresis electrodes. J Electrostat 66:514–525
Lapizco-Encinas BH, Simmons BA, Cummings EB, Fintschenko Y (2004) Insulator-based dielectrophoresis for the selective concentration and separation of live bacteria in water. Electrophoresis 25:1695–1704
Lewpiriyawong N, Yang C, Lam YC (2010) Continuous sorting and separation of microparticles by size using AC dielectrophoresis in a PDMS microfluidic device with 3-D conducting PDMS composite electrodes. Electrophoresis 31:2622–2631
Lewpiriyawong N, Kandaswamy K, Yang C, Ivanov V, Stocker R (2011) Microfluidic characterization and continuous separation of cells and particles using conducting poly(dimethyl siloxane) electrode induced alternating current-dielectrophoresis. Anal Chem 83:9579–9585
Li WH, Du H, Chen DF, Shu C (2004) Analysis of dielectrophoretic electrode arrays for nanoparticle manipulation. Comput Mater Sci 30:320–325
Ling SH (2013) Particle manipulation using dielectrophoresis with microelectrode array. Dissertation, Nanyang Technological University
Ling SH, Lam YC, Kua CH (2011) Particle streaming and separation using dielectrophoresis through discrete periodic microelectrode array. Microfluid Nanofluid 11:579–591
Ling SH, Lam YC, Chian KS (2012) Continuous cell separation using dielectrophoresis through asymmetric and periodic microelectrode array. Anal Chem 84:6463–6470
Martínez G, Sanchis A, Sancho M (2008) Numerical analysis of microelectrodes for cell electrokinetic applications. J Appl Phys 104:064512
Moon HS, Kwon K, Kim SI, Han H, Sohn J, Lee S, Jung HI (2011) Continuous separation of breast cancer cells from blood samples using multi-orifice flow fractionation (MOFF) and dielectrophoresis (DEP). Lab Chip 11:1118–1125
Morgan H, Green NG (2001) AC electrokinetics: colloids and nanoparticles. Research Studies Press, Philadelphia
Morgan H, Alberto García I, David B, Green NG, Antonio R (2001) The dielectrophoretic and travelling wave forces generated by interdigitated electrode arrays: analytical solution using Fourier series. J Phys D Appl Phys 34:1553
Piacentini N, Mernier G, Tornay R, Renaud P (2011) Separation of platelets from other blood cells in continuous-flow by dielectrophoresis field-flow-fractionation. Biomicrofluidics 5:034122–034128
Sun T, Morgan H, Green NG (2007) Analytical solutions of ac electrokinetics in interdigitated electrode arrays: electric field, dielectrophoretic and traveling-wave dielectrophoretic forces. Phys Rev E 76:046610
Wang X et al (1996) A theoretical method of electrical field analysis for dielectrophoretic electrode arrays using Green’s theorem. J Phys D Appl Phys 29:1649
Wang L, Lu J, Marchenko SA, Monuki ES, Flanagan LA, Lee AP (2009) Dual frequency dielectrophoresis with interdigitated sidewall electrodes for microfluidic flow-through separation of beads and cells. Electrophoresis 30:782–791
Acknowledgments
Ling Siang Hooi gratefully acknowledges the financial support of Nanyang Technological University in the form of a NTU Research Scholarship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix 1: Model for E simulation
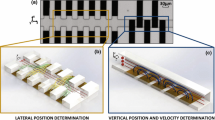
The theoretical model, as shown in Fig. 2a, has dimensions of 1,104.7 µm × 1,003.4 µm × 32 µm (length, L × width, W × height, H). It is constructed with a continuous top electrode and an array of 9 × 9 discrete bottom microelectrodes. The microelectrodes are modeled with dimensions and arrangement as shown in Fig. 1c, and at 1.2 µm below the model bottom surface to match the actual design. The setting of 200 µm for the spacing between the closest microelectrode and the respective sidewall (l) is to minimize boundary effect caused by the sidewalls.
Applying quasi-electrostatic approximation, the distribution of E (\(= - \nabla \Phi\), where \(\Phi\) is the electrical potential) for the model domain could be obtained by solving the Laplace’s equation \(\left( {\nabla \cdot \nabla\Phi = 0} \right)\). With voltage drop over the top and bottom ITO conductors (under experimental conditions at f = 250 kHz and σ f = 503.4 µS/cm), the effective voltage across the DEP buffer in the microchannel (∆V Channel ) over the electrode space was computed to be 62.0 % V SEP (simulation results not shown). Hence, with V SEP of 2.5 and 3.5 VP in the experiments, the corresponding ∆V Channel was 1.55 and 2.17 VP, respectively. With these considerations, the boundary conditions to the model are as follows:
-
\(\Phi =\Delta \varvec{V}_{{\varvec{Channel}}}\) to all triangular red filled areas on the model bottom surface with each area representing a single discrete microelectrode.
-
\(\Phi = 0\) to the model top surface representing the continuous top electrode.
-
\(\nabla \cdot\Phi = 0\) to all other surfaces representing the insulating boundaries. These surfaces include the 1.2-µm-thick edge surfaces of microelectrodes, the bottom surface of the model and the sidewalls.
To assess the grid independency of the E solutions, the model was meshed under: (1) a coarse mesh with ~3.527 × 107 elements and (2) a fine mesh with ~5.555 × 107 elements. With the maximum differences in E between the two meshed cases less than 3.06 % for all z-elevations studied, the accuracy presented by the coarse mesh is therefore acceptable.
Appendix 2: Unit-cell domains
Figure 6 presents the six unit-cell domains. The geometries, dimensions and locations for each unit-cell domain defined in the theoretical model are described as follows:
-
Unit-cell A, as shown in Fig. 6a, has its domain corner at (x, y, z) = (0 µm, 544.7 µm, 0 µm) with dimensions of 432.5 µm × 400 µm × 32 µm (L × W × H). Its domain includes the space with the upper left 3 × 3 microelectrodes of the 9 × 9 microelectrodes.
-
Unit-cell B, as shown in Fig. 6b, has its domain corner at (x, y, z) = (0 µm, 487.7 µm, 0 µm) with dimensions of 432.5 µm × 60 µm × 32 µm (L × W × H). Its domain includes the space with the 1 × 3 microelectrodes on the 4th row across from the 1st to the 3rd columns of the 9 × 9 microelectrodes.
-
Unit-cell C, as shown in Fig. 6c, has its domain corner at (x, y, z) = (432.5 µm, 580.7 µm, 0 µm) with dimensions of 80 µm × 400 µm × 32 µm (L × W × H). Its domain includes the space with the 3 × 1 microelectrodes on the 4th column across from the 1st to the 3rd rows of the 9 × 9 microelectrodes.
-
Unit-cell D, as shown in Fig. 6d, has its domain corner at (x, y, z) = (432.5 µm, 520.7 µm, 0 µm) with dimensions of 80 µm × 60 µm × 32 µm (L × W × H). Its domain includes the space with the single microelectrode on the 4th row and the 4th column of the 9 × 9 microelectrodes.
-
Unit-cell E, as shown in Fig. 6e, has its domain corner at (x, y, z) = (672.5 µm, 613.7 µm, 0 µm) with dimensions of 430 µm × 380 µm × 32 µm (L × W × H). Its domain includes the space with the upper right 3 × 3 microelectrodes of the 9 × 9 microelectrodes.
-
Unit-cell F, as shown in Fig. 6f, has its domain corner at (x, y, z) = (672.5 µm, 553.7 µm, 0 µm) with dimensions of 430 µm × 60 µm × 32 µm (L × W × H). Its domain includes the space with the 1 × 3 microelectrodes on the 4th row across from the 7th to the 9th columns of the 9 × 9 microelectrodes.
Appendix 3: Analysis on t z and t x
The approach to estimate t z and t x is as follows:
-
(a)
For a cell at the assumed z-elevation (z i ) within the space in a unit-cell D (i.e., a repeating unit-cell of the theoretical model), the \(\overline{{\left\langle {{\varvec{F}}_{{{\mathbf{DEP\_}}\varvec{z}}} } \right\rangle }} |_{{z_{i} }}\) on the cell is first calculated. \(\overline{{\left\langle {{\varvec{F}}_{{{\mathbf{DEP\_}}\varvec{z}}} } \right\rangle }} |_{{z_{i} }}\) denotes the averaged z-directional dielectrophoretic force over the x–y plane of the unit-cell D at z i and is expressed as:
$$\overline{{\left\langle {{\varvec{F}}_{{{\mathbf{DEP\_}}\varvec{z}}} } \right\rangle }} |_{{z_{i} }} = \pi \varepsilon_{\text{f}} R^{3} \text{Re} \left[ {f_{{\text{cm}}} } \right]\left( {\overline{{\frac{\partial }{\partial z}\left| \varvec{E} \right|^{2} }} |_{{z_{i} }} } \right),\quad \text{where}\quad \overline{{\;\frac{\partial }{\partial z}\left| \varvec{E} \right|^{2} }} |_{{z_{i} }} = \frac{{\sum\nolimits_{x = i}^{m} {\sum\nolimits_{y = j}^{n} {\left( {\frac{\partial }{\partial z}\left| \varvec{E} \right|^{2} } \right)_{{x,y,z_{i} }} } } }}{{m{ \times }n}}$$(11) -
(b)
The averaged z-directional velocity at z i for the cell \(\left( {\overline{{w_{\text{c}} }} |_{{z_{i} }} } \right)\) is then calculated by dividing the resultant z-component force on the cell by the F Stokes coefficient \(\left( {f = 6\pi \eta RK_{z} } \right)\):
$$\overline{{w_{\text{c}} }} |_{{z_{i} }} = \left( {\frac{{\left( {\rho_{\text{f}} - \rho_{\text{c}} } \right)\text{V}_{\text{c}} \overset{\lower0.5em\hbox{$\smash{\scriptscriptstyle\rightharpoonup}$}} {g} + \overline{{\left\langle {{\varvec{F}}_{{{\mathbf{DEP\_}}\varvec{z}}} } \right\rangle }} |_{{z_{i} }} }}{{6\pi \eta RK_{z} |_{{z_{i} }} }}} \right)$$(12)where \(K_{z} |_{{z_{i} }}\) is the F Stokes correction factor for the cell levitated at z i .
-
(c)
The required time (t j ) for a cell experiencing an averaged positive z-component force to ascend from a lower z-elevation z l to a higher z-elevation (z h ) or a cell experiencing an averaged negative z-component force to descend from z h to z l can be estimated as:
$$\begin{aligned} t_{j} & = \left| {\frac{{z_{l} - z_{h} }}{{\overline{{w_{\text{c}} }} |_{{z_{l} }} }}} \right|\,\text{for cell experiencing an averaged positive}\, z \text{-component force} \\ & = \left| {\frac{{z_{h} - z_{l} }}{{\overline{{w_{\text{c}} }} |_{{z_{h} }} }}} \right|\,\text{for cell experiencing an averaged negative}\, z \text{-component force} \\ \end{aligned} $$(13) -
(d)
The time (t z ) taken for the cell experiences an averaged positive/negative z-component force to travel from the lowest/highest possible z-elevation to the highest/lowest possible z-elevation (i.e., assuming a minimum gap of 1.1R between the cell center and microchannel top/bottom surface) is estimated as:
$$t_{z} = \sum\limits_{{}}^{{}} {t_{j} }$$(14) -
(e)
The time for cell of a similar type to traverse over 100 columns of microelectrode array (t x ) is estimated by taking the average time for twelve cells of a similar type traversing the first 32 microelectrode columns in the experimental images and multiples by \(\frac{100}{32}\). From experimental images on the separation of live/dead NIH-3T3 cells, t x for live and dead cells, respectively, were 9.27 and 5.10 s under V SEP of 2.5 VP, and 14.01 and 5.00 s, respectively, under V SEP of 3.5 VP.
-
(f)
By comparing \(\frac{{t_{z} }}{{t_{x} }}\), the timescale for a cell in descent or ascent with respect to that in traversing over the microelectrode array can be obtained. If \(\frac{{t_{z} }}{{t_{x} }} \to 0\,\%\), the cell is considered to settle instantaneously upon entering the microelectrode region and traverses the microelectrode array at the settled z-elevation (z s). If \(\frac{{t_{z} }}{{t_{x} }}\) is large, the cell settles gradually and only reaches the presumed z s after it traverses a substantial number of microelectrode columns (or may not even settle at the presumed z s even it has traversed the entire microelectrode array if \(\frac{{t_{z} }}{{t_{x} }} \ge 100\%\)). In such scenario, the cell traverses microelectrode array with changing z-elevation.
Figure 7 presents t z for live and dead cells that descend from z i of 1.1R below the microchannel top surface to z i of 1.1R above the microchannel bottom surface, under ∆V Channel of: (a) 1.55 VP, and (b) 2.17 VP. The descent for live cell is rapid, with t z of 0.637 and 1.098 s for 2.17 VP and 1.55 VP, respectively. A smaller t z at increased ∆V Channel is attributed to a stronger positive \(\overline{{\left\langle {{\varvec{F}}_{{{\mathbf{DEP\_}}\varvec{z}}} } \right\rangle }} |_{{z_{i} }}\) on the live cell. For dead cell with neutral DEP behavior, its descent is much more gradual than that of the live cell, with t z of 7.301 s under 1.55 VP and 7.483 s under 2.17 VP.
With the highest and lowest ∆V Channel applied, \(\frac{{t_{z} }}{{t_{x} }}\) for live cells were only 4.55 and 11.84 %, respectively. This implies that live cells traverse most of the microelectrode array with z-elevations near to the microchannel bottom surface. With \(\frac{{t_{z} }}{{t_{x} }} \ge 143.16\,\%\) for dead cells, this signifies that dead cells would traverse the microelectrode array at many possible z-elevations, with its z-elevation strongly depends on its initial z-elevation.
Rights and permissions
About this article
Cite this article
Lam, Y.C., Ling, S.H., Chan, W.Y. et al. Dielectrophoretic cell motion model over periodic microelectrodes with unit-cell approach. Microfluid Nanofluid 18, 873–885 (2015). https://doi.org/10.1007/s10404-014-1478-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-014-1478-8