Abstract
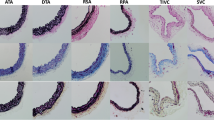
This study assessed the long-term effect of vagotomy on the structure and passive mechanical characteristics of the thoracic aorta under a wide range of stresses in vitro. Eight healthy Landrace pigs underwent bilateral vagotomy distal to the origin of the recurrent laryngeal nerve, and 10 pigs were sham-operated. Three months post-surgery, the aorta was excised and specimens from the ascending aorta, arch, and descending thoracic aorta were subjected to histomorphometrical evaluation and uniaxial tensile-testing until failure. Elastic modulus-stress data were plotted and submitted to regression analysis. Structural remodeling after vagotomy was characterized as vascular growth in the ascending aorta and arch, and as thinning in the descending thoracic aorta. In the aortic segments of vagotomized animals, the area density of elastin and collagen was increased, but smooth muscle density was decreased. Similar differences in regression parameters and failure strength between groups were found in all aortic segments, indicating that the vessel wall was stiffer and stronger in vagotomized animals. In the clinical setting, disease states or drugs blocking the regulatory role of the vagi nerves on the aortic wall may have undesirable consequences on the mechanical performance of the thoracic aorta, and therefore on hemodynamic homeostasis.
Similar content being viewed by others
References
Abbaud, F. M. Disturbances in Neurogenic Control of the Circulation. Baltimore: Williams and Wilkins, 1981.
Adrian, R. H. Reviews in Physiology, Biochemistry and Pharmacology. New York: Springer-Verlag, 1978.
Ahlgren, A. R., G. Sundkvist, P. Wollmer, B. Sonesson, and T. Lanne. Increased aortic stiffness in women with type I diabetes mellitus is associated with diabetes duration and autonomic nerve function. Diabet. Med. 16:291–297, 1999.
Amenta, F., C. Cavallotti, F. Ferrante, and M. Zomparelli. The cholinergic innervation of the aorta. Acta Histochem. 66:197–203, 1980.
Angouras, D., D. P. Sokolis, T. Dosios, N. Kostomitsopoulos, H. Boudoulas, Gr. Skalkeas, and P. E. Karayannacos. Effect of impaired vasa vasorum flow on the structure and mechanics of the thoracic aorta: Implications for the pathogenesis of aortic dissection. Eur. J. Cardiothorac. Surg. 17:468–473, 2000.
Aviado, D. Pharmacology of Ganglionic Transmission. New York: Springer-Verlag, 1979.
Baumbach, G. L., D. D. Heistad, and J. E. Siems. Effect of sympathetic nerves on composition and distensibility of cerebral arterioles in rats. J. Physiol. 416:123–140, 1989.
Bevan, R. D., and H. Tsuru. Functional and structural changes in the rabbit ear artery after sympathetic denervation. Circ. Res. 49:478–485, 1981.
Bhagot, B. Mode of Action of Autonomic Drugs. New York: Graceway, 1979.
Boudoulas, H., and C. F. Wooley. Aortic distensibility. Important in clinical medicine? Cardiol. Rev. 2:211–217, 1994.
Boudoulas, H., and C. F. Wooley. Aortic function. In: Functional Abnormalities of the Aorta, edited by H. Boudoulas, P. K. Toutouzas, and C. F. Wooley. New York: Futura, 1996, pp. 3–36.
Boutouyrie, P., P. Lacolley, X. Girerd, L. Beck, M. Safar, and S. Laurent. Sympathetic activation decreases medium-size artery compliance in humans. Am. J. Physiol. 267:H1368–H1376, 1994.
Cheng, Z., T. L. Powley, J. S. Schwaber, and F. J. Doyle 3rd. A laser confocal microscopic study of vagal afferent innervation of rat aortic arch: Chemoreceptors as well as baroreceptors. J. Auton. Nerv. Syst. 67:1–14, 1997.
Clark, J. M., and S. Glagov. Structural integration of the arterial wall. I. Relationships and attachments of medial smooth muscle cells in normally distended and hyperdistended aortas. Lab. Invest. 40:587–602, 1979.
Clark, J. M., and S. Glagov. Transmural organization of the arterial media. The lamellar unit revisited. Arteriosclerosis 5:19–34, 1985.
Cox, R. H. Mechanics of canine iliac artery smooth muscle in vitro. Am. J. Physiol. 230:H462–H470, 1976.
Cox, R. H. Passive mechanics and connective tissue composition of canine arteries. Am. J. Physiol. 234:H533–H541, 1978.
Dobrin, P. B., and T. Canfield. Elastase, collagenase, and the biaxial elastic properties of dog carotid artery. Am. J. Physiol. 237:H124–H131, 1984.
Dobrin, P. B., and A. A. Rovick. Influence of vascular smooth muscle on contractile mechanics and elasticity of arteries. Am. J. Physiol. 217:1644–1651, 1969.
Donald, D. E., and J. T. Shepherd. Autonomic regulation of the peripheral circulation. Annu. Rev. Physiol. 42:429–439, 1980.
Ferrari, A. U. Age-related modifications in neural cardiovascular control. Aging (Milano) 4:183–195, 1992.
Ferrari, A. U. Modifications of the cardiovascular system with aging. Am. J. Geriatr. Cardiol. 11:30–33, 2002.
Ferrari, A. U., A. Radaelli, and M. Centola. Invited review: Aging and the cardiovascular system. J. Appl. Physiol. 95:2591–2597, 2003.
Fronek, K., C. M. Bloor, D. Amiel, and M. Chvapil. Effect of long-term sympathectomy on the arterial wall in rabbits and rats. Exp. Mol. Pathol. 28:279–289, 1978.
Fung, Y. C. Elasticity of soft tissues in simple elongation. Am. J. Physiol. 213:1532–1544, 1967.
Fung, Y. C. Biorheology of soft tissues. Biorheology 10:139–155, 1973.
Fung, Y. C. Description of internal deformation and forces. In: Biomechanics: Motion, Flow, Stress and Growth, edited by Y. C. Fung. New York: Springer-Verlag, 1990, pp. 353–381.
Glagov, S., and H. Wolinsky. Physiology: Aortic wall as a ‘two phase’ material. Nature 199:606–608, 1963.
Gray, H., and H. V. Carter. Nervous system. In: Gray's Anatomy, edited by H. Gray and H. V. Carter. London: Magpie, 1993, pp. 509–514.
Hayashi, K. Experimental approaches on measuring the mechanical properties and constitutive laws of arterial walls. J. Biomech. Eng. 115:481–488, 1993.
Hayashi, K., T. Washizu, N. Tsushima, R. J. Kirali, and Y. Nose. Mechanical properties of aortas and pulmonary arteries of calves implanted with cardiac prostheses. J. Biomech. 14:173–182, 1981.
Humphrey, J. D. Cardiovascular Solid Mechanics: Cells, Tissues, and Organs, 1st ed. New York: Springer-Verlag, 2002, pp. 68–105.
Lacolley, P., Y. Bezie, X. Girerd, P. Challande, A. Benetos, P. Boutouyrie, N. Ghodsi, B. Lucet, R. Azoui, and S. Laurent. Aortic distensibility and structural changes in sinoaortic-denervated rats. Hypertension 269:H407–H416, 1995.
Lacolley, P., E. Glaser, P. Challande, P. Boutouyrie, J. P. Mignot, M. Duriez, B. Levy, M. Safar, and S. Laurent. Structural changes and in situ aortic pressure-diameter relationship in long-term chemical-sympathectomized rats. Am. J. Physiol. 269:H407–H416, 1995.
Larson, E. W., and W. D. Edwards. Risk factors for aortic dissection: A necropsy study of 161 cases. Am. J. Cardiol. 53:849–855, 1984.
Miao, C. Y., X. Tao, K. Gong, S. H. Zhang, Z. X. Chu, and F. Su. Arterial remodeling in chronic sinoaortic-denervated rats. J. Cardiovasc. Pharmacol. 37:6–15, 2001
Mircoli L., A. A. Mangoni, C. Giannattasio, G. Mancia, and A. U. Ferrari. Heart-rate dependent stiffening of large arteries in intact and sympathectomized rats. Hypertension 32:735–739, 1999.
Pfeifer, M. A., C. R. Weinberg, D. Cook, J. D. Best, A. Reenan, and J. B. Halter. Differential changes of autonomic nervous system function with age in man. Am. J. Med. 75:249–258, 1983.
Roach, M. R., and A. C. Burton. The reason for the shape of the distensibility curves of arteries. Can. J. Biochem. 35:681–690, 1957.
Sokolis, D. P., H. Boudoulas, and P. E. Karayannacos. Assessment of the aortic stress-strain relation in uniaxial tension. J. Biomech. 35:1213–1223, 2002.
Sokolis, D. P., H. Boudoulas, N. Kavantzas, N. Kostomitsopoulos, H. Boudoulas, Gr. Skalkeas, and P. E. Karayannacos. A morphometric study of the structural characteristics of the aorta in pigs using an image analysis method. Anat. Histol. Embryol. 31:1–10, 2002.
Stefanadis, C., C. F. Wooley, C. A. Bush, A. J. Kolibash, and H. Boudoulas. Aortic distensibility abnormalities in coronary artery disease. Am. J. Cardiol. 59:1300–1304, 1987.
Todd, M. E., and B. Gowen. Arterial wall and smooth muscle cell development in young Wistar rats and the effects of surgical denervation. Circ. Res. 69:438–446, 1991.
Wolinsky, H., and S. Glagov. Structural basis for the static mechanical properties of the aortic media. Circ. Res. 14:400–413, 1964.
Zar, J. H. Biostatistical Analysis, 3rd ed. New Jersey: Prentice-Hall, 1996.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sokolis, D.P., Zarbis, N., Dosios, T. et al. Post-Vagotomy Mechanical Characteristics and Structure of the Thoracic Aortic Wall. Ann Biomed Eng 33, 1504–1516 (2005). https://doi.org/10.1007/s10439-005-7118-4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10439-005-7118-4