Abstract
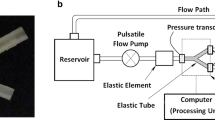
There is a spatial disposition to atherosclerosis along the aorta corresponding to regions of flow disturbances. The objective of the present study is to investigate the detailed distribution of hemodynamic parameters (wall shear stress (WSS), spatial gradient of wall shear stress (WSSG), and oscillatory shear index (OSI)) in the entire length of C57BL/6 mouse aorta with all primary branches (from ascending aorta to common iliac bifurcation). The detailed geometrical parameters (e.g., diameter and length of the vessels) were obtained from casts of entire aorta and primary branches of mice. The flow velocity was measured at the inlet of ascending aorta using Doppler flowprobe in mice. The outlet pressure boundary condition was estimated based on scaling law. The continuity and Navier–Stokes equations were solved using three-dimensional finite element method (FEM). The model prediction was tested by comparing the computed flow rate with the flow rate measured just before the common iliac bifurcation, and good agreement was found. It was also found that complex flow patterns occur at bifurcations between main trunk and branches. The major branches of terminal aorta, with the highest proportion of atherosclerosis, have the lowest WSS, and the relatively atherosclerotic-prone aortic arch has much more complex WSS distribution and higher OSI value than other sites. The low WSS coincides with the high OSI, which approximately obeys a power law relationship. Furthermore, the scaling law between flow and diameter holds in the entire aorta and primary branches of mice under pulsatile blood flow conditions. This model will eventually serve to elucidate the causal relation between hemodynamic patterns and atherogenesis in KO mice.









Similar content being viewed by others
References
Bao X., C. Lu, J. A. Frangos. Mechanism of temporal gradients in shear-induced ERK1/2 activation and proliferation in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 281:H22–H29, 2001
Bentzon J. F., C. S. Sondergaard, M. Kassem, E. Falk. Smooth muscle cells healing atherosclerotic plaque disruptions are of local, not blood, origin in apolipoprotein E knockout mice. Circulation 116(18):2053–2061, 2007
Bentzon J. F., C. Weile, C. S. Sondergaard, J. Hindkjaer, M. Kassem, E. Falk. Smooth muscle cells in atherosclerosis originate from the local vessel wall and not circulating progenitor cells in ApoE knockout mice. Arterioscler. Thromb. Vasc. Biol. 26(12):2696–702, 2006
Buchanan J. R. Jr., C. Kleinstreuer, G. A. Truskey, M. Lei. Relation between non-uniform hemodynamics and sites of altered permeability and lesion growth at the rabbit aorto-celiac junction. Atherosclerosis 143:27–40, 1999
Caro C. G., J. M. Fitz-Gerald, R. C. Schroter. Atheroma and arterial wall shear observations, correlation and proposal of a shear dependent mass transfer mechanism for atherogenesis. Proc. R. Soc. Lond. B. 177:109–159, 1971
Cheng C., D. Tempel, R. van Haperen, A. van der Baan, F. Grosveld, M. J. Daemen, R. Krams, R. de Crom. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 113:2744–2753, 2006
Debakey M. E., G. M. Lawrie, D. H. Glaeser. Patterns of atherosclerosis and their surgical significance. Annl. Surg. 201:115–131, 1985
Feintuch A., P. Ruengsakulrach, A. Lin, J. Zhang, Y. Q. Zhou, J. Bishop, L. Davidson, D. Courtman, F. S. Foster, D. A. Steinman, R. M. Henkelman, C. R. Ethier. Hemodynamics in the mouse aortic arch as assessed by MRI, ultrasound, and numerical modeling. Am. J. Physiol. Heart Circ. Physiol. 292:H884–H892, 2007
Fry D. L. Acute vascular endothelia changes associated with increased blood velocity gradients. Circ. Res. 22:165–197, 1968
Greve J. M., A. S. Les, B. T. Tang, M. T. D. Blomme, N. M. Wilson, R. L. Dalman, N. J. Pelc, C. A. Taylor. Allometric scaling of wall shear stress from mice to humans: quantification using cine phase-contrast MRI and computational fluid dynamics. Am. J. Physiol. Heart Circ. Physiol. 291:H1700–H1708, 2006
Guo X., Y. Kono, R. Mattrey, G. S. Kassab. Morphometry and strain distribution of the C57BL/6 mouse aorta. Am. J. Physiol. Heart Circ. Physiol. 283:H1829–H1837, 2002
Guo X., G. S. Kassab. Variation of mechanical properties along the length of the aorta in C57BL/6 mice. Am. J. Physiol. Heart Circ. Physiol. 285:H2614–H2622, 2003
Guo X., G. S. Kassab. Distribution of stress and strain along the porcine aorta and coronary arterial tree. Am. J. Physiol. Heart Circ. Physiol. 286:H2361–H2368, 2004
Herrmann R. A., R. A. Malinauskas, G. A. Truskey. Characterization of sites of elevated low density lipoprotein at the intercostals celiac and iliac branches of the rabbit aorta. Arterioscler. Thromb. Vasc. Biol. 14:313–323, 1994
Huo Y., G. S. Kassab. Flow patterns in three-dimensional porcine epicardial coronary arterial tree. Am. J. Physiol. Heart Circ. Physiol. 293:H2959–H2970, 2007
Huo Y., B. Q. Li. Surface deformation and marangoni convection in electrostatically-positioned droplets of immiscible liquids under microgravity. ASME J. Heat Trans. 128:520–529, 2006
Huo Y., B. Q. Li. Three-dimensional marangoni convection in electrostatically positioned droplets under microgravity. Int. J. Heat Mass Trans. 47:3533–3547, 2004
Janssen B., J. Debets, P. Leenders, J. Smits. Chronic measurement of cardiac output in conscious mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282:R928–R935, 2002
Johns C., I. Gavras, D. E. Handy, A. Salomao, H. Gavras. Models of experimental hypertension in mice. Hypertension 28:1064–1069, 1996
Kassab G. S. Scaling laws of vascular trees of form and function. Am. J. Physiol. Heart Circ. Physiol. 290:H894–H903, 2006
Kassab G. S. Design of coronary circulation: a minimum energy hypothesis. Comput. Method Appl. Mech. Eng. 196:3033–3042, 2007
Kassab G. S., C. A. Rider, N. J. Tang, Y. C. Fung. Morphometry of pig coronary arterial trees. Am. J. Physiol. Heart Circ. Physiol. 265:H350–H365, 1993
Krivitski N. M., D. Starostin, T. L. Smith. Extracorporeal recording of mouse hemodynamic parameters by ultrasound velocity dilution. ASAIO J. 45:32–36, 1999
Ku D. N., D. P. Gidden, C. K. Zarins, S. Glagov. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Arteriosclerosis 5:293–302, 1985
Lu X., G. S. Kassab. Nitric oxide is significantly reduced in ex vivo pig arteries during reverse flow because of increased superoxide production. J. Physiol. 561(20):575–582, 2004
Malek A. M., S. L. Alper, S. Izumo. Hemodynamic shear stress and its role in atherosclerosis. JAMA 282:2035–2042, 1999
Mattson D. L. Comparison of arterial blood pressure in different strains of mice. Am. J. Hypertens. 14:405–408, 2001
Moore J. E., C. Xu, S. Glagov, G. K. Zarins, D. N. Ku. Fluid wall shear stress measurements in a model of the human abdominal aorta: oscillatory behavior and relationship to atherosclerosis. Atherosclerosis 110:225–240, 1994
Murray C. D. The physiological principle of minimum work applied to the angle of branching of arteries. J. Gen. Physiol. 9:835–841, 1926
Nakashima Y., A. S. Plump, E. W. Raines, J. L. Breslow, R. Ross. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb. Vasc. Biol. 14:133–140, 1992
Nichols W. W., M. F. O’Rourke. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. 4th Ed. New York: Oxford University Press, 1998
Plump A. S., J. D. Smith, T. Hayek, K. Aalto-Setaa, A. Walsh, J. G. Verstuyft, E. M. Rubbin, J. L. Breslow. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 71:343–353, 1992
Pries A. R., D. Neuhaus, P. Gaehtgens. Blood viscosity in tube flow: dependence on diameter and hematocrit. Am. J. Physiol. Heart Circ. Physiol. 163:H1770–H1778, 1992
Schwartz S. M., Z. S. Galis, M. E. Rosendeld, E. Falk. Plaque rupture in humans and mice. Arterioscler. Thromb. Vasc. Biol. 27:705–713, 2007
Shyy J. Y., S. Chien. Role of integrins in endothelial machanosensing of shear stress. Circ. Res. 91:769–775, 2002
Suo J., D. E. Ferrara, D. Sorescu, R. E. Guldberg, W. R. Taylor, D. P. Giddens. Hemodynamic shear stress in mouse aortas implications for atherogenesis. Arterioscler. Thromb. Vasc. Biol. 27:346–351, 2007
Tangirala R. K., E. M. Rubin, W. Palinski. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J. Lipid Res. 36:2320–2328, 1995
Traub O., B. C. Berk. Laminar shear stress mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler. Thromb. Vasc. Biol. 18:677–685, 1998
White C. R., M. Haidekker, X. Bao, J. A. Frangos. Temporal gradients in shear, but not spatial gradients, stimulate endothelial cell proliferation. Circulation 103:2508–2513, 2001
Zeindler C. M., R. G. Kratky, M. R. Roach. Quantitative measurements of early atherosclerotic lesions on rabbit aortae from vascular casts. Atherosclerosis 76:245–255, 1989
Zhang S. H., R. L. Reddick, J. A. Piedrahita, N. Maeda. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein. Science 258:468–471, 1992
Acknowledgments
We thank Carlos O. Linares for providing the technical help. This research is supported in part by the National Institute of Health-National Heart, Lung, and Blood Institute Grant 2 R01 HL055554-11.
Author information
Authors and Affiliations
Corresponding author
Appendix A
Appendix A
Hemodynamic Parameters
The Reynolds (Re), Womersley (α), and Dean (D n ) numbers are defined, respectively, in main trunk of mouse aorta as follows:
where \( V = V_{{\min }} ,{\text{ }}V_{{\max }} ,{\text{ or }}V_{{{\text{mean}}}} \), R and D, ω, ρ, and μ represent minimum, maximum, or time-averaged velocity at the inlet of ascending aorta, radius and diameter of ascending aorta, angular frequency of beating hearts, blood mass density, and viscosity, respectively.
At any point of 3D FEM model, the stress can be represented as a nine-component tensor (\( \ifmmode\expandafter\bar\else\expandafter\=\fi{\ifmmode\expandafter\bar\else\expandafter\=\fi{\tau }} \)), which can be written as follows:
where \( \overline{\overline{D}} = 0.5 \cdot {[ {(\nabla {\mathbf{v}}) + (\nabla {\mathbf{v}})^{{\text{T}}} }]}\) is the shear rate tensor. The stress on the wall, its normal component, and it two tangential components can be written as, respectively:
where n, t 1 , and t 2 are the unit vector in the normal and two tangential directions, respectively. The present time-averaged OSI can be written as follows:
The spatial derivatives of the stress can be obtained as follows:
where n, t 1, and t 2 are the natural coordinates. As define by Buchanan et al.,4 the diagonal components \( \partial \tau _{t_{1}}/ \partial t_{1} \) and \( \partial \tau _{t_{2}} / \partial t_{2}\) generate intracellular tension, which causes widening and shrinking of cellular gap. However, the diagonal component \( \partial \tau _{n}/{\partial n} \) can cause endothelial cells rotation, which may destroy the endothelial function too. Therefore, WSSG is defined as follows:
The time-averaged WSSG can be written:
To plot the shear stress in the entire computational domain, WSS is determined as the product of viscosity (μ) and wall shear rate (\( \ifmmode\expandafter\dot\else\expandafter\.\fi{\gamma } \)), which is defined as:
The time-averaged WSS can be written as follows:
Equations (A6) and (A8–A11) were used to calculate the OSI, WSSG, and WSS in the FEM model.
Rights and permissions
About this article
Cite this article
Huo, Y., Guo, X. & Kassab, G.S. The Flow Field along the Entire Length of Mouse Aorta and Primary Branches. Ann Biomed Eng 36, 685–699 (2008). https://doi.org/10.1007/s10439-008-9473-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-008-9473-4