Abstract
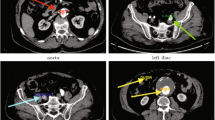
Abdominal Aortic Aneurysms (AAAs), i.e., focal enlargements of the aorta in the abdomen are frequently observed in the elderly population and their rupture is highly mortal. An intra-luminal thrombus is found in nearly all aneurysms of clinically relevant size and multiply affects the underlying wall. However, from a biomechanical perspective thrombus development and its relation to aneurysm rupture is still not clearly understood. In order to explore the impact of blood flow on thrombus development, normal aortas (n = 4), fusiform AAAs (n = 3), and saccular AAAs (n = 2) were compared on the basis of unsteady Computational Fluid Dynamics simulations. To this end patient-specific luminal geometries were segmented from Computerized Tomography Angiography data and five full heart cycles using physiologically realistic boundary conditions were analyzed. Simulations were carried out with computational grids of about half a million finite volume elements and the Carreau–Yasuda model captured the non-Newtonian behavior of blood. In contrast to the normal aorta the flow in aneurysm was highly disturbed and, particularly right after the neck, flow separation involving regions of high streaming velocities and high shear stresses were observed. Naturally, at the expanded sites of the aneurysm average flow velocity and wall shear stress were much lower compared to normal aortas. These findings suggest platelets activation right after the neck, i.e., within zones of pronounced recirculation, and platelet adhesion, i.e., thrombus formation, downstream. This mechanism is supported by recirculation zones promoting the advection of activated platelets to the wall.







Similar content being viewed by others
References
Auer, M., and T. C. Gasser. Reconstruction and finite element mesh generation of abdominal aortic aneurysms from computerized tomography angiography data with minimal user interaction. IEEE T. Med. Imaging 2009 (submitted).
Bird, R. B., R. C. Armstrong, and O. Hassager. Dynamics of Polymeric Liquids, Vol. 1. Fluid Mechanics. New York: Wiley, 1987.
Bluestein, D., L. Niu, R. T. Schoephoerster, and M. K. Dewanjee. Steady flow in an aneurysm model: correlation between fluid dynamics and blood platelet deposition. J. Biomed. Eng. 118:280–286, 1996.
Bluestein, D., L. Niu, R. Schoephoerster, and M. K. Dewanjee. Fluid mechanics of arterial stenosis: relationship to the development of mural thrombus. Ann. Biomed. Eng. 25:344–356, 1997.
Carmo, M., L. Colombo, A. Bruno, F. R. Corsi, L. Roncoroni, M. S. Cuttin, F. Radice, E. Mussini, and P. G. Settembrini. Alteration of elastin, collagen and their cross-links in abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 23:543–549, 2002.
Chen, J., X. Lu, and W. Wang. Non-newtonian effects of blood flow on hemodynamics in distal vascular graft anastomoses. J. Biomech. 39:1983–1995, 2006.
Choke, E., G. Cockerill, W. R. Wilson, S. Sayed, J. Dawson, I. Loftus, and M. M. Thompson. A review of biological factors implicated in abdominal aortic aneurysm rupture. Eur. J. Vasc. Endovasc. Surg. 30:227–244, 2005.
di Martino, E. S., S. Mantero, F. Inzoli, G. Melissano, D. Astore, R. Chiesa, and R. Fumero. Biomechanics of abdominal aortic aneurysm in the presence of endoluminal thrombus: experimental characterization and structural static computational analysis. Eur. J. Vasc. Endovasc. Surg. 15:290–299, 1998.
Fillinger, M. F., M. L. Raghavanand, S. P. Marra, J.-L. Cronenwett, and F. E. Kennedy. In vivo analysis of mechanical wall stress and abdominal aortic aneurysm rupture risk. J. Vasc. Surg. 36:589–597, 2002.
Fleming, C., E. P. Whitlock, T. Beil, and F. A. Lederle. Review: screening for abdominal aortic aneurysm: a best-evidence systematic review for the U.S. preventive services task force. Ann. Intern. Med. 142:203–211, 2005.
Gasser, T. C., G. Görgülü, M. Folkesson, and J. Swedenborg. Failure properties of intraluminal thrombus in abdominal aortic aneurysm under static and pulsating mechanical loads. J. Vasc. Surg. 48:179–188, 2008.
Gasser, T. C., P. Gudmundson, and G. Dohr. Failure mechanisms of ventricular tissue due to deep penetration. J. Biomech. 42:626–633, 2009.
Gijsen, F. J. H., E. Allanic, F. N. van de Vosse, and J. D. Janssen. The influence of the non-Newtonian properties of blood on the flow in large arteries: unsteady flow in a 90° curved tube. J. Biomech. 32:705–713, 1999.
Hans, S. S., O. Jareunpoon, M. Balasubramaniam, and G. B. Zelenock. Size and location of thrombus in intact and ruptured abdominal aortic aneurysms. J. Vasc. Surg. 41:584–588, 2005.
Hellums, J. D. 1993 Whitaker lecture: biorheology in thrombosis research. Ann. Biomed. Eng. 22:445–455, 1994.
Heng, M. S., M. J. Fagan, J. W. Collier, G. Desai, P. T. McCollum, and I. C. Chetter. Peak wall stress measurement in elective and acute abdominal aortic aneurysms. J. Vasc. Surg. 47:17–22, 2008.
Humphrey, J. D., and C. A. Taylor. Intracranial and abdominal aortic aneurysms: similarities, differences, and need for a new class of computational models. Ann. Biomed. Eng. 10:221–246, 2008.
Inzoli, F., F. Boschetti, M. Zappa, T. Longo, and R. Fumero. Biomechanical factors in abdominal aortic aneurysm rupture. Eur. J. Vasc. Surg. 7:667–674, 1993.
Kazi, M., J. Thyberg, P. Religa, J. Roy, P. Eriksson, U. Hedin, and J. Swedenborg. Influence of intraluminal thrombus on structural and cellular composition of abdominal aortic aneurysm wall. J. Vasc. Surg. 38:1283–1292, 2003.
Kazi, M., C. Zhu, J. Roy, G. Paulsson-Berne, A. Hamsten, J. Swedenborg, U. Hedin, and P. Eriksson. Difference in matrix-degrading protease expression and activity between thrombus-free and thrombus-covered wall of abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 25:1341–1346, 2005.
Länne, T., B. Sonesson, D. Bergqvist, H. Bengtsson, and D. Gustafsson. Diameter and compliance in the male human abdominal aorta: influence of age and aortic aneurysm. Eur. J. Vasc. Surg. 6:178–184, 1992.
Leung, J. H., A. R. Wright, N. Cheshire, J. Crane, S. A. Thom, A. D. Hughes, and Y. Xu. Fluid structure interaction of patient specific abdominal aortic aneurysms: a comparison with solid stress models. Biomed. Eng. Online 5–33, 2006.
Li, Z., and C. Kleinstreuer. Computational analysis of type II endoleaks in a stented abdominal aortic aneurysm model. J. Biomech. 2005.
Li, Z., and C. Kleinstreuer. A comparison between different asymmetric abdominal aortic aneurysm morphologies employing computational fluid-structure interaction analysis. Eur. J. Mech. B/Fluids 26:615–631, 2007.
Li, Z.-Y., J. U-King-Im, T. Y. Tang, E. Soh, T. C. See, and J. H. Gillard. Impact of calcification and intraluminal thrombus on the computed wall stresses of abdominal aortic aneurysm. J. Vasc. Surg. 47:928–935, 2008.
Long, A., L. Rouet, A. Bissery, P. Rossignol, D. Mouradian, and M. Sapoval. Compliance of abdominal aortic aneurysms: evaluation of tissue doppler imaging. Ultrasound Med. Biol. 30:1099–1108, 2004.
Macedo, T. A., A. W. Stanson, G. S. Oderich, C. M. Johnson, J. M. Panneton, and M. L. Tie. Infected aortic aneurysms: imaging findings. Radiology 231:250–257, 2004.
Malvern, L. E. Introduction to the Mechanics of a Continuous Medium. Englewood Cliffs, NJ: Prentice-Hall, 1969.
Mills, C., I. Gabe, J. Gault, D. Mason, J. Ross, E. Braunwald, et al. Pressure-flow relationships and vascular impedance in man. Cardiovasc. Res. 4:405–417, 1970.
Mower, W. R., W. J. Quiñones, and S. S. Gambhir. Effect of intraluminal thrombus on abdominal aortic aneurysm wall stress. J. Vasc. Surg. 33:602–608, 1997.
Prakash, S., and C. R. Ethier. Requirements for mesh resolution in 3D computational hemodynamics. J. Biomech. Eng. 123:134–144, 2001.
Raz, S., S. Einav, Y. Alemu, and D. Bluestein. DPIV prediction of flow induced platelet activation—comparison to numerical predictions. Ann. Biomed. Eng. 35:493–504, 2007.
Richardson, P. D. Biomechanics of plaque rupture: progress, problems, and new frontiers. Ann. Biomed. Eng. 30:524–536, 2002.
Rissland, P., Y. Alemu, S. Einav, J. Ricotta, and D. Bluestein. Abdominal aortic aneurysm risk of rupture: patient-specific FSI simulations using anisotropic model. J. Biomech. Eng. 131, 2009 (online).
Sack, J.-R., and J. Urrutia, editors. Handbook of Computational Geometry. Amsterdam: Elsevier, 2000.
Scotti, C. M., and E. A. Finol. Compliant biomechanics of abdominal aortic aneurysms: a fluid-structure interaction study. Comput. Struct. 85:1097–1113, 2007.
Steinman, D. A., D. A. Vorp, and C. R. Ethier. Computational modeling of arterial biomechanics: insights into pathogenesis and treatment of vascular disease. J. Vasc. Res. 37:1118–1128, 2003.
Stenbaek, J., B. Kalin, and J. Swedenborg. Growth of thrombus may be a better predictor of rupture than diameter in patients with abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 20:466–499, 2000.
Swedenborg, J., and P. Eriksson. The intraluminal thrombus as a source of proteolytic activity. Ann. N.Y. Acad. Sci. 1085:133–138, 2006.
Taylor, R. L. FEAP—A Finite Element Analysis Program, Version 7.4 User Manual. Berkeley, California: University of California at Berkeley, 2002.
Upchurch Jr., G. R., and T. A. Schaub. Abdominal aortic aneurysm. Am. Fam. Physician 73:1198–1204, 2006.
van Dam, E. A., S. D. Dams, G. W. M. Peters, M. C. M. Rutten, G. W. H. Schurink, J. Buth, and F. N. van de Vosse. Non-linear viscoelastic behavior of abdominal aortic aneurysm thrombus. Biomech. Model. Mechanobiol. 7:127–137, 2007.
Vande Geest, J. P., M. S. Sacks, and D. A. Vorp. A planar biaxial constitutive relation for the luminal layer of intra-luminal thrombus in abdominal aortic aneurysms. J. Biomech. 39:2347–2354, 2006.
Venkatasubramaniam, A. K., M. J. Fagan, T. Mehta, K. J. Mylankal, B. Ray, G. Kuhan, I. C. Chetter, and P. T. McCollum. A comparative study of aortic wall stress using finite element analysis for ruptured and non-ruptured abdominal aortic aneurysms. Eur. J. Vasc. Surg. 28:168–176, 2004.
Vorp, D. A., P. C. Lee, D. H. Wang, M. S. Makaroun, E. M. Nemoto, S. Ogawa, and M. W. Webster. Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J. Vasc. Surg. 34:291–299, 2001.
Vorp, D. A., W. A. Mandarino, M. W. Webster, and J. Gorcsan. Potential influence of intraluminal thrombus on abdominal aortic aneurysm as assessed by a new non-invasive method. Cardiovasc. Surg. 4:732–739, 1996.
Wang, D. H., M. S. Makaroun, M. W. Webster, and D. A. Vorp. Mechanical properties and microstructure of intraluminal thrombus from abdominal aortic aneurysm. J. Biomech. Eng. 123:536–539, 2001.
Wang, D. H., M. S. Makaroun, M. W. Webster, and D. A. Vorp. Effect of intraluminal thrombus on wall stress in patient-specific models of abdominal aortic aneurysm. J. Vasc. Surg. 36:598–604, 2002.
Wolters, B. J. B. M., M. C. M. Rutten, G. W. H. Schurink, U. Kose, J. de Hart, and F. N. van de Vosse. A patient-specific computational model of fluid-structure interaction in abdominal aortic aneurysms. Med. Eng. Phys. 27:871–883, 2005.
Wootton, D. M., and D. N. Ku. Fluid mechanics of vascular systems, diseases, and thrombosis. Ann. Rev. Biomed. Eng. 1:299–329, 1999.
Xu, C., D. L. Pham, and J. L. Prince. Image segmentation using deformable models. In: Handbook of Medical Imaging. Volume 2. Medical Image Processing and Analysis, edited by M. Sonka and J. M. Fitzpatrick. Bellingham: Spie press, 2000, pp. 129–174.
Acknowledgments
We’d like to thank Dr. Piergiorgio Spazzini (INRiM, Italy) for all the helpful discussions about solving procedure and data analysis. This work has been supported by the Young Faculty Grant No. 2006-7568 provided by the Swedish Research Council, VINNOVA and the Swedish Foundation for Strategic Research, and the EC Seventh Framework Programme, Fighting Aneurysmal Disease (FAD-200647) which is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Biasetti, J., Gasser, T.C., Auer, M. et al. Hemodynamics of the Normal Aorta Compared to Fusiform and Saccular Abdominal Aortic Aneurysms with Emphasis on a Potential Thrombus Formation Mechanism. Ann Biomed Eng 38, 380–390 (2010). https://doi.org/10.1007/s10439-009-9843-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-009-9843-6