Abstract
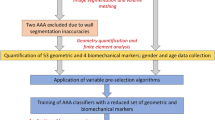
Recent studies have shown that the maximum transverse diameter of an abdominal aortic aneurysm (AAA) and expansion rate are not entirely reliable indicators of rupture potential. We hypothesize that aneurysm morphology and wall thickness are more predictive of rupture risk and can be the deciding factors in the clinical management of the disease. A non-invasive, image-based evaluation of AAA shape was implemented on a retrospective study of 10 ruptured and 66 unruptured aneurysms. Three-dimensional models were generated from segmented, contrast-enhanced computed tomography images. Geometric indices and regional variations in wall thickness were estimated based on novel segmentation algorithms. A model was created using a J48 decision tree algorithm and its performance was assessed using ten-fold cross validation. Feature selection was performed using the χ2-test. The model correctly classified 65 datasets and had an average prediction accuracy of 86.6% (κ = 0.37). The highest ranked features were sac length, sac height, volume, surface area, maximum diameter, bulge height, and intra-luminal thrombus volume. Given that individual AAAs have complex shapes with local changes in surface curvature and wall thickness, the assessment of AAA rupture risk should be based on the accurate quantification of aneurysmal sac shape and size.




Similar content being viewed by others
References
Batchelor, P. G., A. D. Castellano Smith, and D. L. G. Hill. Proper scales of shapes—a curved scale space. LNCS 1682:375–385, 1999.
Batchelor, P. G., A. D. Castellano Smith, D. L. Hill, D. J. Hawkes, T. C. Cox, and A. F. Dean. Measures of folding applied to the development of the human fetal brain. IEEE Trans. Med. Imaging 21:953–965, 2002.
Belkin, M., M. C. Donaldson, and A. D. Whittemore. Abdominal aortic aneurysms. Curr. Opin. Cardiol. 9:581–590, 1994.
Di Martino, E. S., A. Bohra, J. P. Vande Geest, N. Gupta, M. Makaroun, and D. A. Vorp. Biomechanical properties of ruptured versus electively repaired abdominal aortic aneurysm wall tissue. J. Vasc. Surg. 43:570–576, 2006.
Doyle, B. J., A. Callanan, P. E. Burke, P. A. Grace, M. T. Walsh, D. A. Vorp, et al. Vessel asymmetry as an additional diagnostic tool in the assessment of abdominal aortic aneurysms. J. Vasc. Surg. 49:443–454, 2009.
Fillinger, M. F., S. P. Marra, M. L. Raghavan, and F. E. Kennedy. Prediction of rupture risk in abdominal aortic aneurysm during observation: wall stress versus diameter. J. Vasc. Surg. 37:724–732, 2003.
Fillinger, M. F., J. Racusin, R. K. Baker, J. L. Cronenwett, A. Teutelink, M. L. Schermerhorn, et al. Anatomic characteristics of ruptured abdominal aortic aneurysm on conventional CT scans: implications for rupture risk. J. Vasc. Surg. 39:1243–1252, 2004.
Fillinger, M. F., M. L. Raghavan, S. P. Marra, J. L. Cronenwett, and F. E. Kennedy. In vivo analysis of mechanical wall stress and abdominal aortic aneurysm rupture risk. J. Vasc. Surg. 36:589–597, 2002.
Giannoglou, G., G. Giannakoulas, J. Soulis, Y. Chatzizisis, T. Perdikides, N. Melas, et al. Predicting the risk of rupture of abdominal aortic aneurysms by utilizing various geometrical parameters: revisiting the diameter criterion. Angiology 57:487–494, 2006.
Greenhalgh, R. M., L. C. Brown, G. P. Kwong, J. T. Powell, and S. G. Thompson. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet 364:843–848, 2004.
Grobman, W. A., and D. M. Stamilio. Methods of clinical prediction. Am. J. Obstet. Gynecol. 194:888–894, 2006.
Hall, M., E. Frank, G. Holmes, B. Pfahringer, P. Reutemann, and I. H. Witten. The WEKA data mining software: an update. SIGKDD Explor. 11:10–18, 2009.
Lederle, F. A., S. E. Wilson, G. R. Johnson, D. B. Reinke, F. N. Littooy, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N. Engl. J. Med. 346:1437–1444, 2002.
Ma, B., R. E. Harbaugh, and M. L. Raghavan. Three-dimensional geometrical characterization of cerebral aneurysms. Ann. Biomed. Eng. 32:264–273, 2004.
Maier, A., M. W. Gee, C. Reeps, J. Pongratz, H. H. Eckstein, and W. A. Wall. A comparison of diameter, wall stress, and rupture potential index for abdominal aortic aneurysm rupture risk prediction. Ann. Biomed. Eng. 38:3124–3134, 2010.
Martufi, G., E. S. Di Martino, C. H. Amon, S. C. Muluk, and E. A. Finol. Three-dimensional geometrical characterization of abdominal aortic aneurysms: image-based wall thickness distribution. J. Biomech. Eng. 131(6):061015, 2009.
McGloughlin, T. M., and B. J. Doyle. New approaches to abdominal aortic aneurysm rupture risk assessment: engineering insights with clinical gain. Arterioscler. Thromb. Vasc. Biol. 30:1687–1694, 2010.
Mower, W., L. Baraff, and J. Sneyd. Stress distribution in vascular aneurysms: factors affecting risk of aneurysm rupture. J. Surg. Res. 55:155–161, 1993.
Nyilas, R. D., S. M. L. Ng, J. Leung, and X. Y. Xu. Towards a new geometric approach to assess the risk of rupture of abdominal aortic aneurysms using patient specific modeling. In: Abstract SBC2005-I21 of Proceedings of the 2005 Summer Bioengineering Conference.
Pappu, S., A. Dardik, H. Tagare, and R. J. Gusberg. Beyond fusiform and saccular: a novel quantitative tortuosity index may help classify aneurysm shape and predict aneurysm rupture potential. Ann. Vasc. Surg. 22:88–97, 2008.
Peduzzi, P., J. Concato, E. Kemper, T. R. Holford, and A. R. Feinstein. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 49:1373–1379, 1996.
Persson, P. O., and G. Strang. A simple mesh generator in MATLAB. SIAM Rev. 46:329–345, 2004.
Raghavan, M. L., B. Ma, and R. E. Harbaugh. Quantified aneurysm shape and rupture risk. J. Neurosurg. 102:355–362, 2005.
Sacks, M. S., D. A. Vorp, M. L. Raghavan, M. P. Federle, and M. W. Webster. In vivo three-dimensional surface geometry of abdominal aortic aneurysms. Ann. Biomed. Eng. 27:469–479, 1999.
Scotti, C. M., and E. A. Finol. Compliant biomechanics of abdominal aortic aneurysms: a fluid-structure interaction study. Comput. Struct. 85:1097–1113, 2007.
Scotti, C. M., J. Jimenez, S. C. Muluk, and E. A. Finol. Wall stress and flow dynamics in abdominal aortic aneurysms: finite element analysis vs. fluid-structure interaction. Comput. Methods Biomech. Biomed. Eng. 11:301–322, 2008.
Scotti, C. M., A. D. Shkolnik, S. C. Muluk, and E. A. Finol. Fluid-structure interaction in abdominal aortic aneurysms: effects of asymmetry and wall thickness. Biomed. Eng. Online 4:64, 2005.
Shum, J., E. S. Di Martino, A. Goldhammer, D. Goldman, L. Acker, G. Patel, et al. Semi-automatic vessel wall detection and quantification of wall thickness in ct images of human abdominal aortic aneurysms. Med. Phys. 37:638–648, 2010.
Shum J, A. Xu, I. Chatnuntawech, and E. A. Finol. An automated methodology for surface mesh generation of abdominal aortic aneurysms. Ann. Biomed. Eng. doi:10.1007/s10439-010-0165-5.
Somkantha, K., and P. Phuangsuwan. Diagnosis of the abdominal aorta aneurysm in magnetic resonance imaging images. In: International Conference on Computer and Information Technology (ICCIT2009), Tokyo, Japan.
Taubin, G. Signal processing approach to fair surface design. In: Proceedings of the 22nd Annual ACM Conference on Computer Graphics and Interactive Techniques, Los Angeles, CA, 9–11 August. New York: ACM, 1995.
Truijers, M., J. A. Pol, L. J. Schultzekool, S. M. van Sterkenburg, M. F. Fillinger, and J. D. Blankensteijn. Wall stress analysis in small asymptomatic, symptomatic and ruptured abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 33:401–407, 2007.
Upchurch, Jr., G. R., and T. A. Schaub. Abdominal aortic aneurysm. Am. Fam. Physician 73:1198–1204, 2006.
Vande Geest, J. P., E. S. Di Martino, A. Bohra, M. S. Makaroun, and D. A. Vorp. A biomechanics-based rupture potential index for abdominal aortic aneurysm risk assessment: demonstrative application. Ann. N. Y. Acad. Sci. 1085:11–21, 2006.
Venkatasubramaniam, A. K., M. J. Fagan, T. Mehta, K. J. Mylankal, B. Ray, G. Kuhan, et al. A comparative study of aortic wall stress using finite element analysis for ruptured and non-ruptured abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 28:168–176, 2004.
Acknowledgments
The authors would like to acknowledge the research funding from the Bill and Melinda Gates Foundation, Carnegie Mellon University’s Biomedical Engineering Department, the John and Claire Bertucci Graduate Fellowship program, and NIH grants R21EB007651, R21EB008804, and R15HL087268. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Carolyn Rose’s assistance in performing the statistical analysis is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Joan Greve oversaw the review of this article.
Appendices
Appendix 1: Definition of Features (i.e., Geometric Indices) Used in the Geometry Quantification Approach
1D Size Indices
- Dmax::
-
Maximum transverse diameter for all cross sections within the AAA sac
- Dneck,p::
-
Proximal neck diameter immediately below the renal arteries
- Dneck,d::
-
Distal neck diameter
Since typically arterial cross sections are non-circular in shape, the definition used for calculating D max, D neck,p, and D neck,d is the fluid mechanics definition for hydraulic diameter:
where A i the cross-sectional area and P i the perimeter of the same cross section.
Hsac is the height of AAA sac, Lsac the centerline length of AAA sac, Hneck the height of AAA neck, Lneck the centerline length of AAA neck, Hb the bulge height, and dc the distance between the lumen centroid and the centroid of the cross section where Dmax is located.
2D Shape Indices
- DHr::
-
The diameter–height ratio, an expression of the fusiform shape of the AAA sac
- DDr::
-
Diameter–diameter ratio
- Hr::
-
Height ratio, an assessment of the relative neck height in comparison with the AAA height
- BL::
-
Bulge location, provides a measure of the relative position of the maximum transverse dimension with respect to the neck
- β::
-
Asymmetry factor
- T::
-
Tortuosity
where d is the Euclidean distance from the centroid of the cross section where Dneck,p is located on the centroid of the cross section at the AAA distal end.
3D Size Indices
- V::
-
Vessel volume
- S::
-
Vessel surface area
- VILT::
-
Volume of intraluminal thrombus contained within AAA sac
- γ::
-
Ratio of AAA ILT volume
3D Shape Indices
- IPR: :
-
Isoperimetric ratio
$$ IPR = {\frac{S}{V^{2/3}}} $$ - NFI::
-
Non-fusiform index
$$ NFI = \frac{\frac{S}{V^{2/3}}}{\frac{S_\text{fusiform}}{V_{\text{fusiform}}{}^{2/3}}} =\frac{IPR}{IPR_{\text{fusiform}}}$$
Second-Order Curvature-Based Indices (Calculation Described in Detail in Martufi et al. 16)
- GAA :
-
Area averaged Gaussian curvature
- MAA :
-
Area averaged Mean curvature
- GLN :
-
L2 norm of the Gaussian curvature
- MLN :
-
L2 norm of the Mean curvature
Wall Thickness Indices
- tw,min::
-
Minimum wall thickness
- tw,max::
-
Maximum wall thickness
- tw,ave::
-
Average wall thickness
Appendix 2: Brief Description of Decision Tree Models
A decision tree is a predictive machine learning model whose structure is determined by the information gain (IG) of the feature values. The IG can be calculated as:
where
where Y the class label, which in this case is the repair status (elective vs. emergent), X the feature, and t the value of the feature. H(Y) represents the entropy prior to splitting on a certain feature and feature value and \( {{H}}\left( {{{Y}}|{{X}}{:}{{t}}} \right) \) represents the conditional entropy after splitting on a feature and feature value. Entropy is a measure of the impurity of an arbitrary collection of examples, and varies between 0 and 1 where 1 indicates that there are an equal number of examples in each group and 0 indicates that all examples are in the same group. The IG is calculated as the different between H(Y) and \( {{H}}\left( {{{Y}}|{{X}}{:}{{t}}} \right) .\) A high IG is desirable as it indicates that the conditional entropy, H(Y|X:t), is low, which translates as a reduction in entropy after splitting on a certain feature and feature value.
The internal nodes of a decision tree denote the different features; the branches between the nodes provide the possible values that these features can have based on the data, while the terminal nodes yield the classification variable (ruptured or unruptured). The algorithm determines the internal nodes and branches by identifying the feature that discriminates the various instances most clearly; such feature is said to have the highest IG.
For the tree displayed in Fig. 4, the feature that provided the highest IG was L sac. Six ruptured aneurysm had L sac > 160.57 mm and all the unruptured AAAs and the remaining four ruptured AAAs had L sac < 160.57 mm. Since the six ruptured AAAs cannot be further divided, the tree terminates at that node. The IG is recalculated using the remaining 66 unruptured AAAs and the 4 ruptured AAAs. These are then further subdivided using the feature that had the highest IG, which in this case was S.
Rights and permissions
About this article
Cite this article
Shum, J., Martufi, G., Di Martino, E. et al. Quantitative Assessment of Abdominal Aortic Aneurysm Geometry. Ann Biomed Eng 39, 277–286 (2011). https://doi.org/10.1007/s10439-010-0175-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-010-0175-3