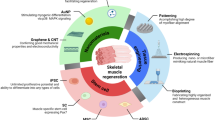
Abstract
The field of tissue engineering involves design of high-fidelity tissue substitutes for predictive experimental assays in vitro and cell-based regenerative therapies in vivo. Design of striated muscle tissues, such as cardiac and skeletal muscle, has been particularly challenging due to a high metabolic demand and complex cellular organization and electromechanical function of the native tissues. Successful engineering of highly functional striated muscles may thus require creation of biomimetic culture conditions involving medium perfusion, electrical and mechanical stimulation. When optimized, these external cues are expected to synergistically and dynamically activate important intracellular signaling pathways leading to accelerated muscle growth and development. This review will discuss the use of different types of tissue culture bioreactors aimed at providing conditions for enhanced structural and functional maturation of engineered striated muscles.



Similar content being viewed by others
References
Aagaard, P., E. B. Simonsen, J. L. Andersen, P. Magnusson, and P. Dyhre-Poulsen. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J. Appl. Physiol. 93:1318–1326, 2002.
Agarwal, A., J. A. Goss, A. Cho, M. L. McCain, and K. K. Parker. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip 13:3599–3608, 2013.
Akar, F. G., R. D. Nass, S. Hahn, E. Cingolani, M. Shah, G. G. Hesketh, D. DiSilvestre, R. S. Tunin, D. A. Kass, and G. F. Tomaselli. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am. J. Physiol. Heart Circ. Physiol. 293:H1223–H1230, 2007.
Akhyari, P., P. W. Fedak, R. D. Weisel, T. Y. Lee, S. Verma, D. A. Mickle, and R. K. Li. Mechanical stretch regimen enhances the formation of bioengineered autologous cardiac muscle grafts. Circulation 106:I137–I142, 2002.
Akins, R. E., N. A. Schroedl, S. R. Gonda, and C. R. Hartzell. 1997. Neonatal rat heart cells cultured in simulated microgravity. In Vitro Cell Dev. Biol. 33:337–343.
Bach, A. D., J. P. Beier, J. Stern-Staeter, and R. E. Horch. Skeletal muscle tissue engineering. J. Cell Mol. Med. 8:413–422, 2004.
Barash, Y., T. Dvir, P. Tandeitnik, E. Ruvinov, H. Guterman, and S. Cohen. Electric field stimulation integrated into perfusion bioreactor for cardiac tissue engineering. Tissue Eng. Part C Methods 16:1417–1426, 2010.
Bardouille, C., J. Lehmann, P. Heimann, and H. Jockusch. Growth and differentiation of permanent and secondary mouse myogenic cell lines on microcarriers. Appl. Microbiol. Biotechnol. 55:556–562, 2001.
Bian, W., and N. Bursac. Tissue engineering of functional skeletal muscle: challenges and recent advances. IEEE Eng. Med. Biol. Mag. 27:109–113, 2008.
Bian, W., B. Liau, N. Badie, and N. Bursac. Mesoscopic hydrogel molding to control the 3D geometry of bioartificial muscle tissues. Nat. Protoc. 4:1522–1534, 2009.
Birla, R. K., Y. C. Huang, and R. G. Dennis. Development of a novel bioreactor for the mechanical loading of tissue-engineered heart muscle. Tissue Eng. 13:2239–2248, 2007.
Boonen, K. J., M. L. Langelaan, R. B. Polak, D. W. van der Schaft, F. P. Baaijens, and M. J. Post. Effects of a combined mechanical stimulation protocol: value for skeletal muscle tissue engineering. J. Biomech. 43:1514–1521, 2010.
Boublik, J., H. Park, M. Radisic, E. Tognana, F. Chen, M. Pei, G. Vunjak-Novakovic, and L. E. Freed. Mechanical properties and remodeling of hybrid cardiac constructs made from heart cells, fibrin, and biodegradable, elastomeric knitted fabric. Tissue Eng. 11:1122–1132, 2005.
Boudou, T., W. R. Legant, A. B. Mu, M. A. Borochin, N. Thavandiran, M. Radisic, P. W. Zandstra, J. A. Epstein, K. B. Margulies, and C. S. Chen. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng. Part A 18:910–919, 2012.
Brevet, A., E. Pinto, J. Peacock, and F. E. Stockdale. Myosin synthesis increased by electrical stimulation of skeletal muscle cell cultures. Science 193:1152–1154, 1976.
Brown, M. A., R. K. Iyer, and M. Radisic. Pulsatile perfusion bioreactor for cardiac tissue engineering. Biotechnol. Prog. 24:907–920, 2008.
Bryant, S. J., J. L. Cuy, K. D. Hauch, and B. D. Ratner. Photo-patterning of porous hydrogels for tissue engineering. Biomaterials 28:2978–2986, 2007.
Buller, A. J., J. C. Eccles, and R. M. Eccles. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J. Physiol. 150:417–439, 1960.
Burkholder, T. J. Mechanotransduction in skeletal muscle. Front Biosci. 12:174–191, 2007.
Bursac, N., Y. Loo, K. Leong, and L. Tung. Novel anisotropic engineered cardiac tissues: studies of electrical propagation. Biochem. Biophys. Res. Commun. 361:847–853, 2007.
Bursac, N., M. Papadaki, R. J. Cohen, F. J. Schoen, S. R. Eisenberg, R. Carrier, G. Vunjak-Novakovic, and L. E. Freed. Cardiac muscle tissue engineering: toward an in vitro model for electrophysiological studies. Am. J. Physiol. 277:H433–H444, 1999.
Bursac, N., M. Papadaki, J. A. White, S. R. Eisenberg, G. Vunjak-Novakovic, and L. E. Freed. Cultivation in rotating bioreactors promotes maintenance of cardiac myocyte electrophysiology and molecular properties. Tissue Eng. 9:1243–1253, 2003.
Candiani, G., S. A. Riboldi, N. Sadr, S. Lorenzoni, P. Neuenschwander, F. M. Montevecchi, and S. Mantero. Cyclic mechanical stimulation favors myosin heavy chain accumulation in engineered skeletal muscle constructs. J. Appl. Biomater. Biomech. 8:68–75, 2010.
Carrier, R. L., M. Papadaki, M. Rupnick, F. J. Schoen, N. Bursac, R. Langer, L. E. Freed, and G. Vunjak-Novakovic. Cardiac tissue engineering: cell seeding, cultivation parameters, and tissue construct characterization. Biotechnol. Bioeng. 64:580–589, 1999.
Carrier, R. L., M. Rupnick, R. Langer, F. J. Schoen, L. E. Freed, and G. Vunjak-Novakovic. Perfusion improves tissue architecture of engineered cardiac muscle. Tissue Eng. 8:175–188, 2002.
Chaudhuri, O., and D. J. Mooney. Stem-cell differentiation: anchoring cell-fate cues. Nat. Mater. 11:568–569, 2012.
Cheema, U., R. Brown, V. Mudera, S. Y. Yang, G. McGrouther, and G. Goldspink. Mechanical signals and IGF-I gene splicing in vitro in relation to development of skeletal muscle. J. Cell. Physiol. 202:67–75, 2005.
Cheema, U., S. Y. Yang, V. Mudera, G. G. Goldspink, and R. A. Brown. 3-D in vitro model of early skeletal muscle development. Cell Motil. Cytoskeleton 54:226–236, 2003.
Chen, H. C., and Y. C. Hu. Bioreactors for tissue engineering. Biotechnol. Lett. 28:1415–1423, 2006.
Chen, M. Q., X. Xie, K. D. Wilson, N. Sun, J. C. Wu, L. Giovangrandi, and G. T. Kovacs. Current-controlled electrical point-source stimulation of embryonic stem cells. Cell. Mol. Bioeng. 2:625–635, 2009.
Cheng, M., M. Moretti, G. C. Engelmayr, and L. E. Freed. Insulin-like growth factor-I and slow, bi-directional perfusion enhance the formation of tissue-engineered cardiac grafts. Tissue Eng. Part A 15:645–653, 2009.
Chopra, A., V. Lin, A. McCollough, S. Atzet, G. D. Prestwich, A. S. Wechsler, M. E. Murray, S. A. Oake, J. Y. Kresh, and P. A. Janmey. Reprogramming cardiomyocyte mechanosensing by crosstalk between integrins and hyaluronic acid receptors. J. Biomech. 45:824–831, 2012.
Chromiak, J. A., J. Shansky, C. Perrone, and H. H. Vandenburgh. Bioreactor perfusion system for the long-term maintenance of tissue-engineered skeletal muscle organoids. In Vitro Cell. Dev. Biol. Anim. 34:694–703, 1998.
Chromiak, J. A., and H. H. Vandenburgh. Glucocorticoid-induced skeletal muscle atrophy in vitro is attenuated by mechanical stimulation. Am. J. Physiol. 262:C1471–C1477, 1992.
Chromiak, J. A., and H. H. Vandenburgh. Mechanical stimulation of skeletal muscle cells mitigates glucocorticoid-induced decreases in prostaglandin production and prostaglandin synthase activity. J. Cell. Physiol. 159:407–414, 1994.
Connor, M. K., I. Irrcher, and D. A. Hood. Contractile activity-induced transcriptional activation of cytochrome C involves Sp1 and is proportional to mitochondrial ATP synthesis in C2C12 muscle cells. J. Biol. Chem. 276:15898–15904, 2001.
Corona, B. T., M. A. Machingal, T. Criswell, M. Vadhavkar, A. C. Dannahower, C. Bergman, W. Zhao, and G. J. Christ. Further development of a tissue engineered muscle repair construct in vitro for enhanced functional recovery following implantation in vivo in a murine model of volumetric muscle loss injury. Tissue Eng. 18(1):1213–1228, 2012.
Costa, K. D., E. J. Lee, and J. W. Holmes. Creating alignment and anisotropy in engineered heart tissue: role of boundary conditions in a model three-dimensional culture system. Tissue Eng. 9:567–577, 2003.
Dennis, R. G., B. Smith, A. Philp, K. Donnelly, and K. Baar. Bioreactors for guiding muscle tissue growth and development. Adv. Biochem. Eng. Biotechnol. 112:39–79, 2009.
Donnelly, K., A. Khodabukus, A. Philp, L. Deldicque, R. G. Dennis, and K. Baar. A novel bioreactor for stimulating skeletal muscle in vitro. Tissue Eng. Part C Methods 16:711–718, 2010.
Dusterhoft, S., and D. Pette. Effects of electrically induced contractile activity on cultured embryonic chick breast muscle cells. Differentiation 44:178–184, 1990.
Engler, A. J., M. A. Griffin, S. Sen, C. G. Bonnemann, H. L. Sweeney, and D. E. Discher. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol. 166:877–887, 2004.
Feng, Z., T. Matsumoto, Y. Nomura, and T. Nakamura. An electro-tensile bioreactor for 3-D culturing of cardiomyocytes. A bioreactor system that simulates the myocardium’s electrical and mechanical response in vivo. IEEE Eng. Med. Biol. Mag. 24:73–79, 2005.
Fink, C., S. Ergun, D. Kralisch, U. Remmers, J. Weil, and T. Eschenhagen. Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement. FASEB J. 14:669–679, 2000.
Flaibani, M., C. Luni, E. Sbalchiero, and N. Elvassore. Flow cytometric cell cycle analysis of muscle precursor cells cultured within 3D scaffolds in a perfusion bioreactor. Biotechnol. Prog. 25:286–295, 2009.
Fredette, B. J., and L. T. Landmesser. A reevaluation of the role of innervation in primary and secondary myogenesis in developing chick muscle. Dev. Biol. 143:19–35, 1991.
Freyssenet, D., M. K. Connor, M. Takahashi, and D. A. Hood. Cytochrome c transcriptional activation and mRNA stability during contractile activity in skeletal muscle. Am. J. Physiol. 277:E26–E32, 1999.
Fuchs, J. R., B. A. Nasseri, and J. P. Vacanti. Tissue engineering: a 21st century solution to surgical reconstruction. Ann. Thorac. Surg. 72:577–591, 2001.
Furuta, A., S. Miyoshi, Y. Itabashi, T. Shimizu, S. Kira, K. Hayakawa, N. Nishiyama, K. Tanimoto, Y. Hagiwara, T. Satoh, K. Fukuda, T. Okano, and S. Ogawa. Pulsatile cardiac tissue grafts using a novel three-dimensional cell sheet manipulation technique functionally integrates with the host heart, in vivo. Circ. Res. 98:705–712, 2006.
Gilbert, P. M., K. L. Havenstrite, K. E. Magnusson, A. Sacco, N. A. Leonardi, P. Kraft, N. K. Nguyen, S. Thrun, M. P. Lutolf, and H. M. Blau. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329:1078–1081, 2010.
Goldspink, G., A. Scutt, P. T. Loughna, D. J. Wells, T. Jaenicke, and G. F. Gerlach. Gene expression in skeletal muscle in response to stretch and force generation. Am. J. Physiol. 262:R356–R363, 1992.
Griffith, L. G., and M. A. Swartz. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 7:211–224, 2006.
Gruver, C. L., F. DeMayo, M. A. Goldstein, and A. R. Means. Targeted developmental overexpression of calmodulin induces proliferative and hypertrophic growth of cardiomyocytes in transgenic mice. Endocrinology 133:376–388, 1993.
Guo, X., M. Gonzalez, M. Stancescu, H. H. Vandenburgh, and J. J. Hickman. Neuromuscular junction formation between human stem cell-derived motoneurons and human skeletal muscle in a defined system. Biomaterials 32:9602–9611, 2011.
Hamalainen, N., and D. Pette. Slow-to-fast transitions in myosin expression of rat soleus muscle by phasic high-frequency stimulation. FEBS Lett. 399:220–222, 1996.
Harris, A. J. Embryonic growth and innervation of rat skeletal muscles. I. Neural regulation of muscle fibre numbers. Philos. Trans. R. Soc. Lond. B Biol. Sci. 293:257–277, 1981.
Harrison, J. H., J. P. Merrill, and J. E. Murray. Renal homotransplantation in identical twins. Surg. Forum 6:432–436, 1956.
Hinds, S., W. Bian, R. G. Dennis, and N. Bursac. The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle. Biomaterials 32:3575–3583, 2011.
Hornberger, T. A., and K. A. Esser. Mechanotransduction and the regulation of protein synthesis in skeletal muscle. Proc. Nutr. Soc. 63:331–335, 2004.
Hsu, Y. H., M. L. Moya, C. C. Hughes, S. C. George, and A. P. Lee. A microfluidic platform for generating large-scale nearly identical human microphysiological vascularized tissue arrays. Lab Chip 13:2990–2998, 2013.
Huang, Y. C., R. G. Dennis, and K. Baar. Cultured slow vs. fast skeletal muscle cells differ in physiology and responsiveness to stimulation. Am. J. Physiol. Cell Physiol. 291:C11–C17, 2006.
Huh, D., Y. S. Torisawa, G. A. Hamilton, H. J. Kim, and D. E. Ingber. Microengineered physiological biomimicry: organs-on-chips. Lab Chip 12:2156–2164, 2012.
Kariya, K., L. R. Karns, and P. C. Simpson. Expression of a constitutively activated mutant of the beta-isozyme of protein kinase C in cardiac myocytes stimulates the promoter of the beta-myosin heavy chain isogene. J. Biol. Chem. 266:10023–10026, 1991.
Kensah, G., I. Gruh, J. Viering, H. Schumann, J. Dahlmann, H. Meyer, D. Skvorc, A. Bar, P. Akhyari, A. Heisterkamp, A. Haverich, and U. Martin. A novel miniaturized multimodal bioreactor for continuous in situ assessment of bioartificial cardiac tissue during stimulation and maturation. Tissue Eng. Part C Methods 17:463–473, 2011.
Kensah, G., A. Roa Lara, J. Dahlmann, R. Zweigerdt, K. Schwanke, J. Hegermann, D. Skvorc, A. Gawol, A. Azizian, S. Wagner, L. S. Maier, A. Krause, G. Drager, M. Ochs, A. Haverich, I. Gruh, and U. Martin. Murine and human pluripotent stem cell-derived cardiac bodies form contractile myocardial tissue in vitro. Eur. Heart J. 34:1134–1146, 2013.
Khodabukus, A., and K. Baar. Defined electrical stimulation emphasizing excitability for the development and testing of engineered skeletal muscle. Tissue Eng. Part C Methods 18:349–357, 2012.
Komuro, I., S. Kudo, T. Yamazaki, Y. Zou, I. Shiojima, and Y. Yazaki. Mechanical stretch activates the stress-activated protein kinases in cardiac myocytes. FASEB J. 10:631–636, 1996.
Kroehne, V., I. Heschel, F. Schugner, D. Lasrich, J. W. Bartsch, and H. Jockusch. Use of a novel collagen matrix with oriented pore structure for muscle cell differentiation in cell culture and in grafts. J. Cell Mol. Med. 12:1582–1838, 2008.
Kudoh, S., H. Akazawa, H. Takano, Y. Zou, H. Toko, T. Nagai, and I. Komuro. Stretch-modulation of second messengers: effects on cardiomyocyte ion transport. Prog. Biophys. Mol. Biol. 82:57–66, 2003.
Kujala, K., A. Ahola, M. Pekkanen-Mattila, L. Ikonen, E. Kerkela, J. Hyttinen, and K. Aalto-Setala. Electrical field stimulation with a novel platform: effect on cardiomyocyte gene expression but not on orientation. Int. J. Biomed. Sci. 8:109–120, 2012.
Kumar, A., R. Murphy, P. Robinson, L. Wei, and A. M. Boriek. Cyclic mechanical strain inhibits skeletal myogenesis through activation of focal adhesion kinase, Rac-1 GTPase, and NF-kappaB transcription factor. FASEB J. 18:1524–1535, 2004.
Freed, L. E., and G. Vunjak-Novakovic. Tissue engineering bioreactors. In Principles of Tissue Engineering. New York: Academic Press, 2000.
Lammerding, J., R. D. Kamm, and R. T. Lee. Mechanotransduction in cardiac myocytes. Ann. N. Y. Acad. Sci. 1015:53–70, 2004.
Lanza, R. P., R. Langer, and J. Vacanti. Principles of tissue engineering. In Principles of Tissue Engineering. San Diego: Academic Press, 2000.
Lasher, R. A., A. Q. Pahnke, J. M. Johnson, F. B. Sachse, and R. W. Hitchcock. Electrical stimulation directs engineered cardiac tissue to an age-matched native phenotype. J. Tissue Eng. 3:1–15, 2012.
Li, Z., X. Guo, S. Matsushita, and J. Guan. Differentiation of cardiosphere-derived cells into a mature cardiac lineage using biodegradable poly(N-isopropylacrylamide) hydrogels. Biomaterials 32:3220–3232, 2011.
Liao, I. C., J. B. Liu, N. Bursac, and K. W. Leong. Effect of electromechanical stimulation on the maturation of myotubes on aligned electrospun fibers. Cell. Mol. Bioeng. 1:133–145, 2008.
Liau, B., N. Christoforou, K. W. Leong, and N. Bursac. Pluripotent stem cell-derived cardiac tissue patch with advanced structure and function. Biomaterials 32:9180–9187, 2011.
Lieu, D. K., J. D. Fu, N. Chiamvimonvat, K. C. Tung, G. P. McNerney, T. Huser, G. Keller, C. W. Kong, and R. A. Li. Mechanism-based facilitated maturation of human pluripotent stem cell-derived cardiomyocytes. Circ. Arrhythm. Electrophysiol. 6:191–201, 2013.
Liu, M., S. Montazeri, T. Jedlovsky, R. Van Wert, J. Zhang, R. K. Li, and J. Yan. Bio-stretch, a computerized cell strain apparatus for three-dimensional organotypic cultures. In Vitro Cell. Dev. Biol. Anim. 35:87–93, 1999.
Loughna, P. T., S. Izumo, G. Goldspink, and B. Nadal-Ginard. Disuse and passive stretch cause rapid alterations in expression of developmental and adult contractile protein genes in skeletal muscle. Development 109:217–223, 1990.
Machingal, M. A., B. T. Corona, T. J. Walters, V. Kesireddy, C. N. Koval, A. Dannahower, W. Zhao, J. J. Yoo, and G. J. Christ. A tissue-engineered muscle repair construct for functional restoration of an irrecoverable muscle injury in a murine model. Tissue Eng. Part A 17:2291–2303, 2011.
Maidhof, R., A. Marsano, E. J. Lee, and G. Vunjak-Novakovic. Perfusion seeding of channeled elastomeric scaffolds with myocytes and endothelial cells for cardiac tissue engineering. Biotechnol. Prog. 26:565–572, 2010.
Maidhof, R., N. Tandon, E. J. Lee, J. Luo, Y. Duan, K. Yeager, E. Konofagou, and G. Vunjak-Novakovic. Biomimetic perfusion and electrical stimulation applied in concert improved the assembly of engineered cardiac tissue. J. Tissue Eng. Regen. Med. 6:e12–e23, 2012.
Maillet, M., J. H. van Berlo, and J. D. Molkentin. Molecular basis of physiological heart growth: fundamental concepts and new players. Nat. Rev. Mol. Cell Biol. 14:38–48, 2013.
Martin, I., D. Wendt, and M. Heberer. The role of bioreactors in tissue engineering. Trends Biotechnol. 22:80–86, 2004.
Martineau, L. C., and P. F. Gardiner. Insight into skeletal muscle mechanotransduction: MAPK activation is quantitatively related to tension. J. Appl. Physiol. 91:693–702, 2001.
Molkentin, J. D. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc. Res. 63:467–475, 2004.
Molkentin, J. D., and G. W. Dorn, 2nd. Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu. Rev. Physiol. 63:391–426, 2001.
Molnar, G., N. A. Schroedl, S. R. Gonda, and C. R. Hartzell. Skeletal muscle satellite cells cultured in simulated microgravity. In Vitro Cell. Dev. Biol. Anim. 33:386–391, 1997.
Moon, D. G., G. Christ, J. D. Stitzel, A. Atala, and J. J. Yoo. Cyclic mechanical preconditioning improves engineered muscle contraction. Tissue Eng. 14(5):848, 2008.
Musi, N., H. Yu, and L. J. Goodyear. AMP-activated protein kinase regulation and action in skeletal muscle during exercise. Biochem. Soc. Trans. 31:191–195, 2003.
Naumann, K., and D. Pette. Effects of chronic stimulation with different impulse patterns on the expression of myosin isoforms in rat myotube cultures. Differentiation 55:203–211, 1994.
Neumann, T., S. D. Hauschka, and J. E. Sanders. Tissue engineering of skeletal muscle using polymer fiber arrays. Tissue Eng. 9:995–1003, 2003.
Nunes, S. S., J. W. Miklas, J. Liu, R. Aschar-Sobbi, Y. Xiao, B. Zhang, J. Jiang, S. Masse, M. Gagliardi, A. Hsieh, N. Thavandiran, M. A. Laflamme, K. Nanthakumar, G. J. Gross, P. H. Backx, G. Keller, and M. Radisic. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat. Methods 10:781–787, 2013.
Okano, T., S. Satoh, T. Oka, and T. Matsuda. Tissue engineering of skeletal muscle. Highly dense, highly oriented hybrid muscular tissues biomimicking native tissues. ASAIO J. 43:M749–M753, 1997.
Otis, J. S., T. J. Burkholder, and G. K. Pavlath. Stretch-induced myoblast proliferation is dependent on the COX2 pathway. Exp. Cell Res. 310:417–425, 2005.
Papadaki, M., N. Bursac, R. Langer, J. Merok, G. Vunjak-Novakovic, and L. E. Freed. Tissue engineering of functional cardiac muscle: molecular, structural, and electrophysiological studies. Am. J. Physiol. Heart Circ. Physiol. 280:H168–H178, 2001.
Perrone, C. E., D. Fenwick-Smith, and H. H. Vandenburgh. Collagen and stretch modulate autocrine secretion of insulin-like growth factor-1 and insulin-like growth factor binding proteins from differentiated skeletal muscle cells. J. Biol. Chem. 270:2099–2106, 1995.
Pette, D. Historical perspectives: plasticity of mammalian skeletal muscle. J. Appl. Physiol. 90:1119–1124, 2001.
Portner, R., S. Nagel-Heyer, C. Goepfert, P. Adamietz, and N. M. Meenen. Bioreactor design for tissue engineering. J. Biosci. Bioeng. 100:235–245, 2005.
Powell, C. A., B. L. Smiley, J. Mills, and H. H. Vandenburgh. Mechanical stimulation improves tissue-engineered human skeletal muscle. Am. J. Physiol. Cell Physiol. 283:C1557–C1565, 2002.
Radisic, M., M. Euloth, L. Yang, R. Langer, L. E. Freed, and G. Vunjak-Novakovic. High-density seeding of myocyte cells for cardiac tissue engineering. Biotechnol. Bioeng. 82:403–414, 2003.
Radisic, M., A. Marsano, R. Maidhof, Y. Wang, and G. Vunjak-Novakovic. Cardiac tissue engineering using perfusion bioreactor systems. Nat. Protoc. 3:719–738, 2008.
Radisic, M., H. Park, F. Chen, J. E. Salazar-Lazzaro, Y. Wang, R. Dennis, R. Langer, L. E. Freed, and G. Vunjak-Novakovic. Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng. 12:2077–2091, 2006.
Radisic, M., H. Park, H. Shing, T. Consi, F. J. Schoen, R. Langer, L. E. Freed, and G. Vunjak-Novakovic. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc. Natl Acad. Sci. U.S.A. 101:18129–18134, 2004.
Ryder, J. W., R. Fahlman, H. Wallberg-Henriksson, D. R. Alessi, A. Krook, and J. R. Zierath. Effect of contraction on mitogen-activated protein kinase signal transduction in skeletal muscle. Involvement Of the mitogen- and stress-activated protein kinase 1. J. Biol. Chem. 275:1457–1462, 2000.
Sadoshima, J., and S. Izumo. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu. Rev. Physiol. 59:551–571, 1997.
Sakaguchi, K., T. Shimizu, S. Horaguchi, H. Sekine, M. Yamato, M. Umezu, and T. Okano. In vitro engineering of vascularized tissue surrogates. Sci. Rep. 3:1316, 2013.
Sathaye, A., N. Bursac, S. Sheehy, and L. Tung. Electrical pacing counteracts intrinsic shortening of action potential duration of neonatal rat ventricular cells in culture. J. Mol. Cell. Cardiol. 41:633–641, 2006.
Sauer, H., G. Rahimi, J. Hescheler, and M. Wartenberg. Effects of electrical fields on cardiomyocyte differentiation of embryonic stem cells. J. Cell. Biochem. 75:710–723, 1999.
Sekine, H., T. Shimizu, K. Sakaguchi, I. Dobashi, M. Wada, M. Yamato, E. Kobayashi, M. Umezu, and T. Okano. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat. Commun. 4:1399, 2013.
Serena, E., M. Flaibani, S. Carnio, L. Boldrin, L. Vitiello, P. De Coppi, and N. Elvassore. Electrophysiologic stimulation improves myogenic potential of muscle precursor cells grown in a 3D collagen scaffold. Neurol. Res. 30:207–214, 2008.
Shansky, J., B. Creswick, P. Lee, X. Wang, and H. Vandenburgh. Paracrine release of insulin-like growth factor 1 from a bioengineered tissue stimulates skeletal muscle growth in vitro. Tissue Eng. 12:1833–1841, 2006.
Shapira-Schweitzer, K., and D. Seliktar. Matrix stiffness affects spontaneous contraction of cardiomyocytes cultured within a PEGylated fibrinogen biomaterial. Acta Biomater. 3:33–41, 2007.
Slentz, D. H., G. A. Truskey, and W. E. Kraus. Effects of chronic exposure to simulated microgravity on skeletal muscle cell proliferation and differentiation. In Vitro Cell. Dev. Biol. Anim. 37:148–156, 2001.
Sung, J. H., M. B. Esch, J. M. Prot, C. J. Long, A. Smith, J. J. Hickman, and M. L. Shuler. Microfabricated mammalian organ systems and their integration into models of whole animals and humans. Lab Chip 13:1201–1212, 2013.
Taber, L. A. Biomechanical growth laws for muscle tissue. J. Theor. Biol. 193:201–213, 1998.
Takahashi, H., T. Shimizu, M. Nakayama, M. Yamato, and T. Okano. The use of anisotropic cell sheets to control orientation during the self-organization of 3D muscle tissue. Biomaterials 34:7372–7380, 2013.
Tidball, J. G. Mechanical signal transduction in skeletal muscle growth and adaptation. J. Appl. Physiol. 98:1900–1908, 2005.
Truskey, G., H. Achneck, N. Bursac, H. F. Chan, C. Cheng, C. Fernandez, S. Hong, Y. Jung, T. Koves, W. Kraus, K. Leong, L. Madden, W. Reichert, and X. Zhao. Design considerations for an integrated microphysiological muscle tissue for drug and tissue toxicity testing. Stem Cell Res. Ther. (in press, 2013).
Tsang, V. L., and S. N. Bhatia. Three-dimensional tissue fabrication. Adv. Drug Deliv. Rev. 56:1635–1647, 2004.
Tulloch, N. L., V. Muskheli, M. V. Razumova, F. S. Korte, M. Regnier, K. D. Hauch, L. Pabon, H. Reinecke, and C. E. Murry. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ. Res. 109:47–59, 2011.
van Berlo, J. H., M. Maillet, and J. D. Molkentin. Signaling effectors underlying pathologic growth and remodeling of the heart. J. Clin. Invest 123:37–45, 2013.
Vandenburgh, H. A computerized mechanical cell stimulator for tissue culture: effects on skeletal muscle organogenesis. In Vitro Cell. Dev. Biol. 24:609–619, 1988.
Vandenburgh, H. H., S. Hatfaludy, P. Karlisch, and J. Shansky. Mechanically induced alterations in cultured skeletal muscle growth. J. Biomech. 24(Suppl 1):91–99, 1991.
Vandenburgh, H. H., P. Karlisch, and L. Farr. Maintenance of highly contractile tissue-cultured avian skeletal myotubes in collagen gel. In Vitro Cell. Dev. Biol. 24:166–174, 1988.
Vandenburgh, H., J. Shansky, F. Benesch-Lee, V. Barbata, J. Reid, L. Thorrez, R. Valentini, and G. Crawford. Drug-screening platform based on the contractility of tissue-engineered muscle. Muscle Nerve 37:438–447, 2008.
Vandenburgh, H. H., J. Shansky, P. Karlisch, and R. L. Solerssi. Mechanical stimulation of skeletal muscle generates lipid-related second messengers by phospholipase activation. J. Cell. Physiol. 155:63–71, 1993.
Wilson, S. J., and A. J. Harris. Formation of myotubes in aneural rat muscles. Dev. Biol. 156:509–518, 1993.
Wretman, C., A. Lionikas, U. Widegren, J. Lannergren, H. Westerblad, and J. Henriksson. Effects of concentric and eccentric contractions on phosphorylation of MAPK(erk1/2) and MAPK(p38) in isolated rat skeletal muscle. J. Physiol. 535:155–164, 2001.
Yeung, E. W., N. P. Whitehead, T. M. Suchyna, P. A. Gottlieb, F. Sachs, and D. G. Allen. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J. Physiol. 562:367–380, 2005.
Zhang, D., I. Y. Shadrin, J. Lam, H. Q. Xian, H. R. Snodgrass, and N. Bursac. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials 34:5813–5820, 2013.
Zimmermann, W. H., K. Schneiderbanger, P. Schubert, M. Didie, F. Munzel, J. F. Heubach, S. Kostin, W. L. Neuhuber, and T. Eschenhagen. Tissue engineering of a differentiated cardiac muscle construct. Circ. Res. 90:223–230, 2002.
Acknoweldgments
This work was supported by NIH-NIAMS Grants AR055226 and AR065873, NIH-NHLBI Grant HL104326, and NIH Common Fund for the Microphysiological Systems Initiative Grant UH2TR000505 to N.B. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Tzung Hsiai oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Rangarajan, S., Madden, L. & Bursac, N. Use of Flow, Electrical, and Mechanical Stimulation to Promote Engineering of Striated Muscles. Ann Biomed Eng 42, 1391–1405 (2014). https://doi.org/10.1007/s10439-013-0966-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-013-0966-4