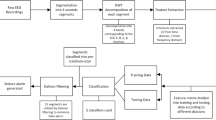
Abstract
Aim of our project is to further optimize neonatal seizure detection using support vector machine (SVM). First, a Kalman filter (KF) was used to filter both feature and classifier output time series in order to increase temporal precision. Second, EEG baseline feature correction (FBC) was introduced to reduce inter patient variability in feature distributions. The performance of the detection methods is evaluated on 54 multi channel routine EEG recordings from 39 both term and pre-term newborns. The area under the receiver operating characteristics curve (AUC) as well as sensitivity and specificity are used to evaluate the performance of the classification method. SVM without KF and FBC achieves an AUC of 0.767 (sensitivity 0.679, specificity 0.707). The highest AUC of 0.902 (sensitivity 0.801, specificity 0.831) is achieved on baseline corrected features with a Kalman smoother used for training data pre-processing and a KF used to filter the classifier output. Both FBC and KF significantly improve neonatal epileptic seizure detection. This paper introduces significant improvements for the state of the art SVM based neonatal epileptic seizure detection.






Similar content being viewed by others
References
Aarabi, A., F. Wallois, and R. Grebe. Automated neonatal seizure detection: a multistage classification system through feature selection based on relevance and redundancy analysis. Clin. Neurophysiol. 117:328–340, 2006.
Aarabi, A., R. Grebe, and F. Wallois. A multistage knowledge-based system for EEG seizure detection in newborn infants. Clin. Neurophysiol. 118:2781–2797, 2007.
Bar-Shalom, Y., T. Kirubarajan, and X.-R. Li. Estimation with Applications to Tracking and Navigation. New York: Wiley, 2002.
Chisci, L., A. Mavino, G. Perferi, M. Sciandrone, C. Anile, G. Colicchio, and F. Fuggetta. Real-time epileptic seizure prediction using AR models and support vector machines. IEEE Trans. Biomed. Eng. 57:1124–1132, 2010.
De Weerd, A. W., P. A. Despland, P. Plouin, and Neonatal EEG. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. 52:149–157, 1999.
Deburchgraeve, W., P. J. Cherian, M. De Vos, R. M. Swarte, J. H. Blok, G. H. Visser, P. Govaert, and S. Van Huffel. Automated neonatal seizure detection mimicking a human observer reading EEG. Clin. Neurophysiol. 119:2447–2454, 2008.
Gotman, J., D. Flanagan, J. Zhang, and B. Rosenblatt. Automatic seizure detection in the newborn: methods and initial evaluation. Electroencephalogr. Clin. Neurophysiol. 103:356–362, 1997.
Greene, B. R., S. Faul, W. P. Marnane, G. Lightbody, I. Korotchikova, and G. B. Boylan. A comparison of quantitative EEG features for neonatal seizure detection. Clin. Neurophysiol. 119:1248–1261, 2008.
Hanley, J. A., and B. J. McNeil. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148:839–843, 1983.
MATLAB version 7.14.0. Natick. Massachusetts: The MathWorks Inc., 2012.
Navakatikyan, M. A., P. B. Colditz, C. J. Burke, T. E. Inder, J. Richmond, and C. E. Williams. Seizure detection algorithm for neonates based on wave-sequence analysis. Clin. Neurophysiol. 117:1190–1203, 2006.
Pachori, R. B., and V. Bajaj. Analysis of normal and epileptic seizure EEG signals using empirical mode decomposition. Comput. Methods Programs Biomed. 104:373–381, 2011.
Rauch, H. E., F. Tung, and C. T. Striebel. Maximum likelihood estimates of linear dynamic systems. AIAA J. 3:1445–1450, 1965.
Scher, M. S., M. Y. Hamid, D. A. Steppe, M. E. Beggarly, and M. J. Painter. Ictal and interictal electrographic seizure durations in preterm and term neonates. Epilepsia 34:284–288, 1993.
Scher, M. S., D. A. Steppe, R. J. Sclabassi, and D. L. Banks. Regional differences in spectral EEG measures between healthy term and preterm infants. Pediatr. Neurol. 17:218–223, 1997.
Scholkopf, B. S. A. Learning with Kernels. Cambridge: MIT press, 2002.
Temko, A., C. Nadeu, W. Marnane, G. B. Boylan, and G. Lightbody. EEG signal description with spectral-envelope-based speech recognition features for detection of neonatal seizures. IEEE Trans. Inf. Technol. Biomed. 15:839–847, 2011.
Temko, A., E. Thomas, W. Marnane, G. Lightbody, and G. Boylan. EEG-based neonatal seizure detection with support vector machines. Clin. Neurophysiol. 122:464–473, 2011.
Temko, A., E. Thomas, W. Marnane, G. Lightbody, and G. B. Boylan. Performance assessment for EEG-based neonatal seizure detectors. Clin. Neurophysiol. 122:474–482, 2011.
Tharp, B. R. Electrophysiological brain maturation in premature infants: an historical perspective. J. Clin. Neurophysiol. 7:302–314, 1990.
Vapnik, V. Estimation of dependences based on empirical data: Springer series in statistics (Springer Series in Statistics). New Yok: Springer, 1982.
Wirrell, E. C., E. A. Armstrong, L. D. Osman, and J. Y. Yager. Prolonged seizures exacerbate perinatal hypoxic-ischemic brain damage. Pediatr. Res. 50:445–454, 2001.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Leonidas D Iasemidis oversaw the review of this article.
Appendix
Appendix
Kalman Filter
It is assumed that epilepsy is observed through the continuous and noisy features z k calculated from the EEG. By modelling the behaviour of z k by means of a state space model the KF can be applied to obtain a filtered version \(\hat{f}_{\text{k}}\) of the noiseless feature value f k. The behaviour of z k can be modelled as the result of a noisy measurement process:
\(z_{\text{k}} = f_{\text{k}} + v_{\text{k}}\) with v k the zero-mean measurement noise with standard deviation σ v. Furthermore it is assumed that f k undergoes smooth transitions between seizure and NS states. This behaviour can be enforced by a white noise acceleration model (WNA) with a nearly-constant rate of change.3 We now introduce the state vector \(s_{\text{k}} = \left[ {f_{\text{k}} ,\dot{f}_{\text{k}} } \right]\) with \(\dot{f}_{\text{k}}\) the rate of change of f k. The signal z k can now be described by the following state-space model:
T represents the sample interval and w k the zero-mean process disturbance with covariance
\(\sigma_{\text{w }}\) is the assumed standard deviation of the random fluctuations of \(\dot{f}_{\text{k}}\). The KF can now be applied to Eq. (1) in order to recursively calculate a filtered estimate \(\hat{f}_{\text{k}}\) of f k. Instead of the calculated feature z k the filtered estimate of the noiseless feature \(\hat{f}_{\text{k}}\) will be used in the training and classification procedure. The gain of the KF only depends on the ratio \(\sigma_{\text{KF}} \triangleq \sigma_{{{\text{w}} }} /\sigma_{{{\text{v}} }}\), \(\sigma_{\text{v}}\) is set to 1 and \(\sigma_{\text{w }}\) is varied from 10−6 to 10−2 (in 9 equally log-spaced steps) in order to tune the filter’s performance. Different optimal filter gains (\(\sigma_{\text{w }}\)) were found for the KF-test (1 × 10−3) and the KS-train (1 × 10−3) in the train phase.
The amount of filtering that is applied by the KF depends on the gain \(\sigma_{\text{w }}\) which is the only parameter that has to be set by the user. The value of \(\sigma_{\text{w }}\)will be varied to tune filter performance.
Rights and permissions
About this article
Cite this article
Bogaarts, J.G., Gommer, E.D., Hilkman, D.M.W. et al. EEG Feature Pre-processing for Neonatal Epileptic Seizure Detection. Ann Biomed Eng 42, 2360–2368 (2014). https://doi.org/10.1007/s10439-014-1089-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-014-1089-2