Abstract
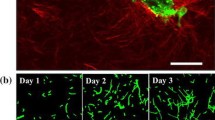
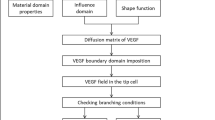
A thorough understanding of determining factors in angiogenesis is a necessary step to control the development of new blood vessels. Extracellular matrix density is known to have a significant influence on cellular behaviors and consequently can regulate vessel formation. The utilization of experimental platforms in combination with numerical models can be a powerful method to explore the mechanisms of new capillary sprout formation. In this study, using an integrative method, the interplay between the matrix density and angiogenesis was investigated. Owing the fact that the extracellular matrix density is a global parameter that can affect other parameters such as pore size, stiffness, cell–matrix adhesion and cross-linking, deeper understanding of the most important biomechanical or biochemical properties of the ECM causing changes in sprout morphogenesis is crucial. Here, we implemented both computational and experimental methods to analyze the mechanisms responsible for the influence of ECM density on the sprout formation that is difficult to be investigated comprehensively using each of these single methods. For this purpose, we first utilized an innovative approach to quantify the correspondence of the simulated collagen fibril density to the collagen density in the experimental part. Comparing the results of the experimental study and computational model led to some considerable achievements. First, we verified the results of the computational model using the experimental results. Then, we reported parameters such as the ratio of proliferating cells to migrating cells that was difficult to obtain from experimental study. Finally, this integrative system led to gain an understanding of the possible mechanisms responsible for the effect of ECM density on angiogenesis. The results showed that stable and long sprouts were observed at an intermediate collagen matrix density of 1.2 and 1.9 mg/ml due to a balance between the number of migrating and proliferating cells. As a result of weaker connections between the cells and matrix, a lower collagen matrix density (0.7 mg/ml) led to unstable and broken sprouts. However, higher matrix density (2.7 mg/ml) suppressed sprout formation due to the high level of matrix entanglement, which inhibited cell migration. This study also showed that extracellular matrix density can influence sprout branching. Our experimental results support this finding.








Similar content being viewed by others
References
Alberts, B., et al. Molecular Biology of the Cell (4th ed.). New York: Garland Science, 2002.
Anderson, A. R. A., and M. A. J. Chaplain. Continuous and discrete mathematical models of tumor-induced angiogenesis. Bull. Math. Biol. 60(5):857–899, 1998.
Asahara, T., et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 85(3):221–228, 1999.
Bauer, A. L., T. L. Jackson, and Y. Jiang. A cell-based model exhibiting branching and anastomosis during tumor-induced angiogenesis. Biophys. J. 92(9):3105–3121, 2007.
Bauer, A. L., T. L. Jackson, and Y. Jiang. Topography of extracellular matrix mediates vascular morphogenesis and migration speeds in angiogenesis. PLoS Comput. Biol. 5(7):e1000445, 2009.
Bauer, A. L., et al. Using sequence-specific chemical and structural properties of DNA to predict transcription factor binding sites. PLoS Comput. Biol. 6(11):e1001007, 2010.
Bauer, A. L., et al. Receptor cross-talk in angiogenesis: mapping environmental cues to cell phenotype using a stochastic, Boolean signaling network model. J. Theor. Biol. 264(3):838–846, 2010.
Bentley, K., M. Jones, and B. Cruys. Predicting the future: towards symbiotic computational and experimental angiogenesis research. Exp. Cell Res. 319(9):1240–1246, 2013.
Boas, S. E. M., et al. Computational modeling of angiogenesis: towards a multi-scale understanding of cell-cell and cell-matrix interactions. Mechanical and Chemical Signaling in Angiogenesis, Berlin: Springer, 2013, pp. 161–183.
Chen, R. R., et al. Integrated approach to designing growth factor delivery systems. FASEB J. 21(14):3896–3903, 2007.
Chung, S., et al. Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Lab Chip 9(2):269–275, 2009.
Cross, V. L., et al. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials 31(33):8596–8607, 2010.
Daub, J. T., and R. M. H. Merks. A cell-based model of extracellular-matrix-guided endothelial cell migration during angiogenesis. Bull. Math. Biol. 75(8):1377–1399, 2013.
Davis, G. E., K. J. Bayless, and A. Mavila. Molecular basis of endothelial cell morphogenesis in three-dimensional extracellular matrices. Anat. Rec. 268(3):252–275, 2002.
De Bock, K., M. Georgiadou, and P. Carmeliet. Role of endothelial cell metabolism in vessel sprouting. Cell Metab. 18(5):634–647, 2013.
Edgar, L. T., et al. Extracellular matrix density regulates the rate of neovessel growth and branching in sprouting angiogenesis. PLoS One 9(1):e85178, 2014.
Edgar, L. T., et al. Mechanical interaction of angiogenic microvessels with the extracellular matrix. J. Biomech. Eng. 136(2):021001, 2014.
Farahat, W. A., et al. Ensemble analysis of angiogenic growth in three-dimensional microfluidic cell cultures. PLoS One 7(5):e37333, 2012.
Ferrara, N., H.-P. Gerber, and J. LeCouter. The biology of VEGF and its receptors. Nat. Med. 9(6):669–676, 2003.
Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1(1):27–30, 1995.
Folkman, J., and P. A. D’Amore. Blood vessel formation: what is its molecular basis? Cell 87(7):1153–1155, 1996.
Friedl, P., and E. B. Bröcker. The biology of cell locomotion within three-dimensional extracellular matrix. Cell. Mol. Life Sci. CMLS 57(1):41–64, 2000.
Gerhardt, H., et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161(6):1163–1177, 2003.
Ghajar, C. M., et al. Mesenchymal stem cells enhance angiogenesis in mechanically viable prevascularized tissues via early matrix metalloproteinase upregulation. Tissue Eng. 12(10):2875–2888, 2006.
Ghajar, C. M., et al. The effect of matrix density on the regulation of 3-D capillary morphogenesis. Biophys. J. 94(5):1930–1941, 2008.
Griffith, L. G., and M. A. Swartz. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 7(3):211–224, 2006.
Hanahan, D., and J. Folkman. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86(3):353–364, 1996.
Helm, C.-L. E., et al. Synergy between interstitial flow and VEGF directs capillary morphogenesis in vitro through a gradient amplification mechanism. Proc. Natl. Acad. Sci. USA 102(44):15779–15784, 2005.
Herbert, S. P., and D. Y. R. Stainier. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat. Rev. Mol. Cell Biol. 12(9):551–564, 2011.
Holash, J., et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 284(5422):1994–1998, 1999.
Irvin, M. W., et al. Techniques and assays for the study of angiogenesis. Exp. Biol Med. 239:1476–1488, 2014.
Jabbarzadeh, E., and C. F. Abrams. Simulations of chemotaxis and random motility in 2D random porous domains. Bull. Math. Biol. 69(2):747–764, 2007.
Jabbarzadeh, E., and C. F. Abrams. Strategies to enhance capillary formation inside biomaterials: a computational study. Tissue Eng. 13(8):2073–2086, 2007.
Jakobsson, L., et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol. 12(10):943–953, 2010.
Jamali, Y., M. Azimi, and M. R. K. Mofrad. A sub-cellular viscoelastic model for cell population mechanics. PLoS One 5(8):e12097, 2010.
Kleinstreuer, N., et al. A computational model predicting disruption of blood vessel development. PLoS Comput. Biol. 9(4):e1002996, 2013.
Kniazeva, E., and A. J. Putnam. Endothelial cell traction and ECM density influence both capillary morphogenesis and maintenance in 3-D. Am. J. Physiol. 297(1):C179–C187, 2009.
Korff, T., and H. G. Augustin. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J. Cell Sci. 112(19):3249–3258, 1999.
Krasik, E. F., and D. A. Hammer. A semianalytic model of leukocyte rolling. Biophys. J. 87(5):2919–2930, 2004.
Kroon, M. E., et al. Collagen type 1 retards tube formation by human microvascular endothelial cells in a fibrin matrix. Angiogenesis 5(4):257–265, 2002.
Liu, J., et al. Angiogenesis activators and inhibitors differentially regulate caveolin-1 expression and caveolae formation in vascular endothelial cells. Angiogenesis inhibitors block vascular endothelial growth factor-induced down-regulation of caveolin-1. J. Biol. Chem. 274(22):15781–15785, 1999.
McDougall, S. R., A. R. A. Anderson, and M. A. J. Chaplain. Mathematical modelling of dynamic adaptive tumour-induced angiogenesis: clinical implications and therapeutic targeting strategies. J. Theor. Biol. 241(3):564–589, 2006.
Mortimer, D., et al. Axon guidance by growth-rate modulation. Proc. Natl. Acad. Sci. 107(11):5202–5207, 2010.
Mousa, S. A., and P. J. Davis. Angiogenesis assays: an appraisal of current techniques. Angiogenesis Modulations in Health and Disease, Dordrecht: Springer, 2013, pp. 1–12.
Moussavi-Baygi, R., et al. Biophysical coarse-grained modeling provides insights into transport through the nuclear pore complex. Biophys. J. 100(6):1410–1419, 2011.
Nakayama, M., et al. Spatial regulation of VEGF receptor endocytosis in angiogenesis. Nat. Cell Biol. 15(3):249–260, 2013.
Nguyen, D.-H. T., et al. Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc. Natl. Acad. Sci. 110(17):6712–6717, 2013.
Peirce, S. M., F. Mac Gabhann, and V. L. Bautch. Integration of experimental and computational approaches to sprouting angiogenesis. Curr. Opin. Hematol. 19(3):184–191, 2012.
Phng, L.-K., F. Stanchi, and H. Gerhardt. Filopodia are dispensable for endothelial tip cell guidance. Development 140(19):4031–4040, 2013.
Qutub, A. A., and A. S. Popel. Elongation, proliferation & migration differentiate endothelial cell phenotypes and determine capillary sprouting. BMC Syst. Biol. 3(1):13, 2009.
Shamloo, A., and S. C. Heilshorn. Matrix density mediates polarization and lumen formation of endothelial sprouts in VEGF gradients. Lab Chip 10(22):3061–3068, 2010.
Shamloo, A., et al. Endothelial cell polarization and chemotaxis in a microfluidic device. Lab Chip 8(8):1292–1299, 2008.
Shin, Y., et al. In vitro 3D collective sprouting angiogenesis under orchestrated ANG-1 and VEGF gradients. Lab Chip 11(13):2175–2181, 2011.
Sieminski, A. L., R. P. Hebbel, and K. J. Gooch. The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Exp. Cell Res. 297(2):574–584, 2004.
Sieminski, A. L., et al. The stiffness of three-dimensional ionic self-assembling peptide gels affects the extent of capillary-like network formation. Cell Biochem. Biophys. 49(2):73–83, 2007.
Smith, Q., and S. Gerecht. Going with the flow: microfluidic platforms in vascular tissue engineering. Curr. Opin. Chem. Eng. 3:42–50, 2014.
Song, J. W., D. Bazou, and L. L. Munn. Anastomosis of endothelial sprouts forms new vessels in a tissue analogue of angiogenesis. Integr. Biol. 4(8):857–862, 2012.
Stokes, C. L., and D. A. Lauffenburger. Analysis of the roles of microvessel endothelial cell random motility and chemotaxis in angiogenesis. J. Theor. Biol. 152(3):377–403, 1991.
Tammela, T., et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature 454(7204):656–660, 2008.
Vasudev, N. S., and A. R. Reynolds. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis 17:471–494, 2014.
Vickerman, V., et al. Design, fabrication and implementation of a novel multi-parameter control microfluidic platform for three-dimensional cell culture and real-time imaging. Lab Chip 8(9):1468–1477, 2008.
Vickerman, V., C. Kim, and R. D. Kamm. Microfluidic devices for angiogenesis. Mechanical and Chemical Signaling in Angiogenesis, Berlin: Springer, 2013, pp. 93–120.
Welti, J., et al. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J. Clin. Investig. 123(8):3190–3200, 2013.
Young, E. W. K. Advances in microfluidic cell culture systems for studying angiogenesis. J. Lab. Autom. 18(6):427–436, 2013.
Zaman, M. H., et al. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc. Natl. Acad. Sci. 103(29):10889–10894, 2006.
Conflict of interest
None Declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael Gower oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shamloo, A., Mohammadaliha, N., Heilshorn, S.C. et al. A Comparative Study of Collagen Matrix Density Effect on Endothelial Sprout Formation Using Experimental and Computational Approaches. Ann Biomed Eng 44, 929–941 (2016). https://doi.org/10.1007/s10439-015-1416-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-015-1416-2