Abstract
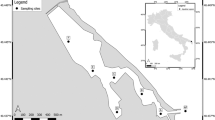
The cyanobacterium Microcystis is notorious for forming extensive and potentially toxic blooms in nutrient-rich freshwater bodies worldwide. However, little is known about the factors underlying the genetic diversity and structure of natural Microcystis populations, despite the fact that this knowledge is essential to understand the build-up of blooms. Microcystis blooms are common and occur year-round in Africa, but are underinvestigated in this continent. We studied the genetic diversity and structure of Microcystis populations in 30 man-made reservoirs in Tigray (Northern Ethiopia) using Denaturing Gradient Gel Electrophoresis of the 16S–23S rDNA internal transcribed spacer (ITS) region and assessed the importance of local environmental conditions and geographic position of the reservoirs for the observed patterns. The analyses showed that both regional and local Microcystis ITS diversity in these recently constructed reservoirs was relatively low, with several dense blooms containing only a single ITS type. Especially one non-toxic ITS type dominated a considerable fraction of Microcystis blooms, but appeared restricted in its geographic distribution. The relationship between Microcystis ITS population structure and abiotic variables (water clarity, pH) and with zooplankton (Daphnia biomass) indicates a (limited) influence of environmental conditions on Microcystis population structure in the reservoirs of Tigray.





Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Ballot A, Pflugmacher S, Wiegand C, Kotut K, Krienitz L (2003) Cyanobacterial toxins in Lake Baringo, Kenya. Limnologica 33:2–9. doi:10.1016/S0075-9511(03)80003-8
Bañares-España E, Lopez-Rodas V, Salgado C, Costas E, Flores-Moya A (2006) Inter-strain variability in the photosynthetic use of inorganic carbon, exemplified by the pH compensation point, in the cyanobacterium Microcystis aeruginosa. Aquat Bot 85:159–162. doi:10.1016/j.aquabot.2006.03.009
Barker GLA, Handley BA, Vacharapiyasophon P, Stevens JR, Hayes PK (2000) Allele-specific PCR shows that genetic exchange occurs among genetically diverse Nodularia (Cyanobacteria) filaments in the Baltic Sea. Microbiology 146:2865–2875
Beisner BE, Haydon DT, Cuddington K (2003) Alternative stable states in ecology. Front Ecol Environ 1:376–382
Bittencourt-Oliveira MD, de Oliveira MC, Bolch CJS (2001) Genetic variability of Brazilian strains of the Microcystis aeruginosa complex (Cyanobacteria/Cyanophyceae) using the phycocyanin intergenic spacer and flanking regions (cpcBA). J Phycol 37:810–818. doi:10.1046/j.1529-8817.2001.00102.x
Bonnet E, Van de Peer Y (2002) Zt: a software tool for simple and partial Mantel tests. J Stat Softw 7:1–12
Bottrell HH, Duncan A, Gliwicz ZM, Grygierek E, Herzig A, Hillbrichtilkowska A, Kurasawa H, Larsson P, Weglenska T (1976) Review of some problems in zooplankton production studies. Norw J Zool 24:419–456
Briand E, Escoffier N, Straub C, Sabart M, Quiblier C, Humbert JF (2009) Spatiotemporal changes in the genetic diversity of a bloom-forming Microcystis aeruginosa (cyanobacteria) population. ISME J 3:419–429
Cadel-Six S, Dauga C, Castets AM, Rippka R, Bouchier C, de Marsac NT, Welker M (2008) Halogenase genes in nonribosomal peptide synthetase gene clusters of Microcystis (Cyanobacteria): sporadic distribution and evolution. Mol Biol Evol 25:2031–2041. doi:10.1093/molbev/msn150
Clarke KR, Gorley RN (2001) PRIMER 5 for windows. PRIMER-E Ltd, Plymouth, UK
Codd GA, Morrison LF, Metcalf JS (2005) Cyanobacterial toxins: risk management for health protection. Toxicol Appl Pharmacol 203:264–272. doi:10.1016/j.taap.2004.02.016
Cohan FM (2002) What are bacterial species? Annu Rev Microbiol 56:457–487. doi:10.1146/annurev.micro.56.012302.160634
Davis TW, Berry DL, Boyer GL, Gobler CJ (2009) The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 8:715–725. doi:10.1016/j.hal.2009.02.004
Dejenie T, Asmelash T, De Meester L, Mulugeta A, Gebrekidan A, Risch S, Pals A, Van der Gucht K, Vyverman W, Nyssen J, Deckers J, Declerck S (2008) Limnological and ecological characteristics of tropical highland reservoirs in Tigray, Northern Ethiopia. Hydrobiologia 610:193–209. doi:10.1007/s10750-008-9435-8
Dejenie T, Asmelash T, Rousseaux S, Gebregiorgis T, Gebrekidan A, Teferi M, Nyssen J, Deckers J, Van der Gucht K, Vyverman W, De Meester L, Declerck S (2009) Impact of the fish Garra on the ecology of reservoirs and the occurrence of Microcystis blooms in semi-arid tropical highlands: an experimental assessment using enclosures. Freshw Biol 54:1605–1615. doi:10.1111/j.1365-2427.2009.02209.x
Drake JA (1991) Community-assembly mechanisms and the structure of an experimental species ensemble. Am Nat 137:1–26
Ferrão-Filho AS, Azevedo S, DeMott WR (2000) Effects of toxic and non-toxic cyanobacteria on the life history of tropical and temperate cladocerans. Freshw Biol 45:1–19. doi:10.1046/j.1365-2427.2000.00613.x
Guillard RR, Lorenzen CJ (1972) Yellow-green algae with chlorophyllide c. J Phycol 8:10–14
Haande S, Ballot A, Rohrlack T, Fastner J, Wiedner C, Edvardsen B (2007) Diversity of Microcystis aeruginosa isolates (Chroococcales, Cyanobacteria) from East-African water bodies. Arch Microbiol 188:15–25. doi:10.1007/s00203-007-0219-8
Hisbergues M, Christiansen G, Rouhiainen L, Sivonen K, Borner T (2003) PCR-based identification of microcystin-producing genotypes of different cyanobacterial genera. Arch Microbiol 180:402–410. doi:10.1007/s00203-003-0605-9
Huisman J, Matthijs HCP, Visser PM (2005) Harmful cyanobacteria. Springer, Dordrecht
Humbert JF, Duris-Latour D, Le Berre B, Giraudet H, Salencon MJ (2005) Genetic diversity in Microcystis populations of a French storage reservoir assessed by sequencing of the 16S–23S rRNA intergenic spacer. Microb Ecol 49:308–314. doi:10.1007/s00248-004-0004-z
Janse I, Meima M, Kardinaal WEA, Zwart G (2003) High-resolution differentiation of cyanobacteria by using rRNA-internal transcribed spacer denaturing gradient gel electrophoresis. Appl Environ Microbiol 69:6634–6643. doi:10.1128/AEM.69.11.6634-6643.2003
Janse I, Kardinaal WEA, Meima M, Fastner J, Visser PM, Zwart G (2004) Toxic and non-toxic Microcystis colonies in natural populations can be differentiated on the basis of rRNA gene internal transcribed spacer diversity. Appl Environ Microbiol 70:3979–3987. doi:10.1128/AEM.70.7.3979-3987.2004
Kardinaal WEA, Janse I, Kamst-van Agterveld M, Meima M, Snoek J, Mur LR, Huisman J, Zwart G, Visser PM (2007a) Microcystis genotype succession in relation to microcystin concentrations in freshwater lakes. Aquat Microb Ecol 48:1–12
Kardinaal WEA, Tonk L, Janse I, Hol S, Slot P, Huisman J, Visser PM (2007b) Competition for light between toxic and nontoxic strains of the harmful cyanobacterium Microcystis. Appl Environ Microbiol 73:2939–2946. doi:10.1128/AEM.02892-06
Lodders N, Stackebrandt E, Nubel U (2005) Frequent genetic recombination in natural populations of the marine cyanobacterium Microcoleus chthonoplastes. Environ Microbiol 7:434–442. doi:10.1111/j.1462-2920.2005.00730.x
Louette G, De Meester L (2007) Predation and priority effects in experimental zooplankton communities. Oikos 116:419–426. doi:10.1111/j.2006.0030-1299.15381.x
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Mantel N, Valand RS (1970) A technique of nonparametric multivariate analysis. Biometrics 26:547–558
Martiny AC, Tai APK, Veneziano D, Primeau F, Chisholm SW (2009) Taxonomic resolution, ecotypes and the biogeography of Prochlorococcus. Environ Microbiol 11:823–832. doi:10.1111/j.1462-2920.2008.01803.x
Maynard Smith J, Smith NH, Orourke M, Spratt BG (1993) How clonal are bacteria? Proc Natl Acad Sci USA 90:4384–4388
Menden-Deuer S, Lessard EJ (2000) Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol Oceanogr 45:569–579
Muyzer G, Dewaal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction amplified genes coding for 16S ribosomal RNA. Appl Environ Microbiol 59:695–700
Oberholster PJ, Myburgh JG, Govender D, Bengis R, Botha AM (2009) Identification of toxigenic Microcystis strains after incidents of wild animal mortalities in the Kruger National Park, South Africa. Ecotox Environ Safe 72:1177–1182. doi:10.1016/j.ecoenv.2008.12.014
Okello W, Ostermaier V, Portmann C, Gademann K, Kurmayer R (2010) Spatial isolation favours the divergence in microcystin net production by Microcystis in Ugandan freshwater lakes. Water Res 44:2803–2814
Otsuka S, Suda S, Li RH, Watanabe M, Oyaizu H, Matsumoto S, Watanabe MM (1999) Phylogenetic relationships between toxic and non-toxic strains of the genus Microcystis based on 16S to 23S internal transcribed spacer sequence. FEMS Microbiol Lett 172:15–21. doi:10.1111/j.1574-6968.1999.tb13443.x
Oudra B, Loudiki M, Sbiyyaa B, Martins R, Vasconcelos V, Namikoshi N (2001) Isolation, characterization and quantification of microcystins (heptapeptides hepatotoxins) in Microcystis aeruginosa dominated bloom of Lalla Takerkoust lake-reservoir (Morocco). Toxicon 39:1375–1381. doi:10.1016/S0041-0101(01)00093-9
Pflugmacher S (2004) Promotion of oxidative stress in the aquatic macrophyte Ceratophyllum demersum during biotransformation of the cyanobacterial toxin microcystin-LR. Aquat Toxicol 70:169–178. doi:10.1016/j.aquatox.2004.06.010
Reche I, Pulido-Villena E, Morales-Baquero R, Casamayor EO (2005) Does ecosystem size determine aquatic bacterial richness? Ecology 86:1715–1722. doi:10.1890/04-1587
Rinta-Kanto JM, Konopko EA, DeBruyn JM, Bourbonniere RA, Boyer GL, Wilhelm SW (2009) Lake Erie Microcystis: relationship between microcystin production, dynamics of genotypes and environmental parameters in a large lake. Harmful Algae 8:665–673. doi:10.1016/j.hal.2008.12.004
Rocap G, Distel DL, Waterbury JB, Chisholm SW (2002) Resolution of Prochlorococcus and Synechococcus ecotypes by using 16S–23S ribosomal DNA internal transcribed spacer sequences. Appl Environ Microbiol 68:1180–1191. doi:10.1128/AEM.68.3.1180-1191.2002
Rohrlack T, Dittmann E, Henning M, Borner T, Kohl JG (1999) Role of microcystins in poisoning and food ingestion inhibition of Daphnia galeata caused by the cyanobacterium Microcystis aeruginosa. Appl Environ Microbiol 65:737–739
SAERT (Sustainable Agricultural and Environmental Rehabilitation in Tigray) (1994) Sustainable agriculture in Tigray. Working document, Mekelle
Rudi K, Skulberg OM, Larsen F, Jakobsen KS (1997) Strain characterization and classification of oxyphotobacteria in clone cultures on the basis of 16S rRNA sequences from the variable regions V6, V7, and V8. Appl Environ Microbiol 63:2593–2599
Sabart M, Pobel D, Latour D, Robin J, Salençon M-J, Humbert J-F (2009) Spatiotemporal changes in the genetic diversity in French bloom-forming populations of the toxic cyanobacterium, Microcystis aeruginosa. Environ Microbiol Reports 1:263–272. doi:10.1111/j.1758-2229.2009.00042.x
Scott WE (1991) Occurrence and significance of toxic cyanobacteria in Southern Africa. Water Sci Technol 23:175–180
Tanabe Y, Kasai F, Watanabe MM (2007) Multilocus sequence typing (MLST) reveals high genetic diversity and clonal population structure of the toxic cyanobacterium Microcystis aeruginosa. Microbiology 153:3695–3703. doi:10.1099/mic.0.2007/010645-0
Vaitomaa J, Rantala A, Halinen K, Rouhiainen L, Tallberg P, Mokelke L, Sivonen K (2003) Quantitative real-time PCR for determination of microcystin synthetase E copy numbers for Microcystis and Anabaena in lakes. Appl Environ Microbiol 69:7289–7297. doi:10.1128/AEM.69.12.7289-7297.2003
van der Oost R (2007) Cyanotoxine monitoring: standaardisering en validatie van methoden voor de Nederlandse waterkwaliteitsbeheerders. Rapport STOWA onderzoek 2005 and 2006. Waternet/Waterproef, Amsterdam
van Gremberghe I, Van Wichelen J, Van der Gucht K, Vanormelingen P, D’Hondt S, Boutte C, Wilmotte A, Vyverman W (2008) Covariation between zooplankton community composition and cyanobacterial community dynamics in Lake Blaarmeersen (Belgium). FEMS Microbiol Ecol 63:222–237. doi:10.1111/j.1574-6941.2007.00422.x
van Gremberghe I, Vanormelingen P, Van der Gucht K, Souffreau C, Vyverman W, De Meester L (2009a) Priority effects in experimental populations of the cyanobacterium Microcystis. Environ Microbiol 11:2564–2573. doi:10.1111/j.1462-2920.2009.01981.x
van Gremberghe I, Vanormelingen P, Vanelslander B, Van der Gucht K, D’hondt S, De Meester L, Vyverman W (2009b) Genotype-dependent interactions among sympatric Microcystis strains mediated by Daphnia grazing. Oikos 118:1647–1658. doi:10.1111/j.1600-0706.2009.17538.x
Van Wichelen J, van Gremberghe I, Vanormelingen P, Menzel D, Descy J-P, Codd J, Vyverman W (2010) Strong grazing effects of amoebae on the biomass and genetic structure of a bloom of the cyanobacterium Microcystis. Environ Microbiol 12:2797–2813. doi:10.1111/j.1462-2920.2010.02249.x
Vézie C, Rapala J, Vaitomaa J, Seitsonen J, Sivonen K (2002) Effect of nitrogen and phosphorus on growth of toxic and nontoxic Microcystis strains and on intracellular microcystin concentrations. Microb Ecol 43:443–454. doi:10.1007/s00248-001-0041-9
Via-Ordorika L, Fastner J, Kurmayer R, Hisbergues M, Dittmann E, Komarek J, Erhard M, Chorus I (2004) Distribution of microcystin-producing and non-microcystin-producing Microcystis sp. in European freshwater bodies: Detection of microcystins and microcystin genes in individual colonies. Syst Appl Microbiol 27:592–602. doi:10.1078/0723202041748163
Welker M, Brunke M, Preussel K, Lippert I, von Dohren H (2004) Diversity and distribution of Microcystis (Cyanobacteria) oligopeptide chemotypes from natural communities studied by single-colony mass spectrometry. Microbiology-SGM 150:1785–1796. doi:10.1099/mic.0.26947-0
Welker M, Sejnohova L, Nemethova D, von Dohren H, Jarkovsky J, Marsalek B (2007) Seasonal shifts in chemotype composition of Microcystis sp communities in the pelagial and the sediment of a shallow reservoir. Limnol Oceanogr 52:609–619. doi:10.4319/lo.2007.52.2.0609
Wilson AE, Sarnelle O, Neilan BA, Salmon TP, Gehringer MM, Hay ME (2005) Genetic variation of the bloom-forming cyanobacterium Microcystis aeruginosa within and among lakes: implications for harmful algal blooms. Appl Environ Microbiol 71:6126–6133. doi:10.1128/AEM.71.10.6126-6133.2005
Wilson AE, Wilson WA, Hay ME (2006) Intraspecific variation in growth and morphology of the bloom-forming cyanobacterium Microcystis aeruginosa. Appl Environ Microbiol 72:7386–7389. doi:10.1128/AEM.00834-06
Wu ZX, Gan NQ, Song LR (2007) Genetic diversity: geographical distribution and toxin profiles of Microcystis strains (Cyanobacteria) in China. J Integr Plant Biol 49:262–269. doi:10.1111/j.1744-7909.2007.00368.x
Yoshida M, Yoshida T, Takashima Y, Hosoda N, Hiroishi S (2007) Dynamics of microcystin-producing and non-microcystin-producing Microcystis populations is correlated with nitrate concentration in a Japanese lake. FEMS Microbiol Lett 266:49–53. doi:10.1111/j.1574-6968.2006.00496.x
Yoshida M, Yoshida T, Satomi M, Takashima Y, Hosoda N, Hiroishi S (2008) Intra-specific phenotypic and genotypic variation in toxic cyanobacterial Microcystis strains. J Appl Microbiol 105:407–415. doi:10.1111/j.1365-2672.2008.03754.x
Zwart G, Huismans R, van Agterveld MP, Van de Peer Y, De Rijk P, Eenhoorn H, Muyzer G, van Hannen EJ, Gons HJ, Laanbroek HJ (1998) Divergent members of the bacterial division Verrucomicrobiales in a temperate freshwater lake. FEMS Microbiol Ecol 25:159–169. doi:10.1016/S0168-6496(97)00092-5
Acknowledgments
This research was financially supported by the Aquatic Ecology project in the VLIR Inter University Cooperation programme with MEKELLE University. We thank the local university authorities, the programme research managers Jan Nyssen and Hans Bauer, and programme director Seppe Deckers for their support. We thank Ann-Eline Debeer for helping with DGGE analyses. The comparison of local ITS diversity to data from Flanders was possible thanks to samples obtained in the framework of the project “B-Blooms” (Algal blooms: emerging problem for health and sustainable use of surface waters), nationally supported by BELSPO (Belgian Science Policy).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Bas W. Ibelings.
Rights and permissions
About this article
Cite this article
van Gremberghe, I., Van der Gucht, K., Vanormelingen, P. et al. Genetic diversity of Microcystis blooms (Cyanobacteria) in recently constructed reservoirs in Tigray (Northern Ethiopia) assessed by rDNA ITS. Aquat Ecol 45, 289–306 (2011). https://doi.org/10.1007/s10452-011-9354-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-011-9354-z