Abstract
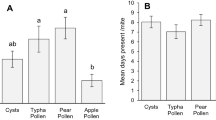
Generalist predators have the potential advantage to control more than one pest and to be more persistent than specialist predators because they can survive on different foods. Moreover, their population growth rate may be elevated when offered a mixture of prey species. We studied a generalist predatory mite Balaustium sp. that shows promise for biological control of thrips and whiteflies in protected rose cultures in Colombia. Although starting its life in the soil, this predator makes excursions onto plants where it feeds on various arthropods. We quantified life history parameters of the predator, offering high densities of three pest species: first-instar larvae of Frankliniella occidentalis, eggs of Trialeurodes vaporariorum and Tetranychus urticae, either alone or in combination. The predators completed their life cycle on each diet. The egg-to-egg period was c. 2 months. All eggs were laid in one batch in 1–2 days, indicating a pronounced semelparous reproduction pattern. In general, females reproduced earlier and laid more eggs on mixed diets, and these early reproducers consequently had higher population growth rates than late reproducers. The best diet in terms of egg-to-egg period and juvenile survival was the combination of eggs from whiteflies and spider mites. Spider mite eggs alone and western flower thrips larvae alone were the worst diets. It remains to be investigated whether mixed diets promote the population growth rate of Balaustium sufficiently for biocontrol of whiteflies and thrips in the presence of alternative prey, such as spider mites, to become effective.






Similar content being viewed by others
References
Bakker FM, Sabelis MW (1989) How larvae of Thrips tabaci reduce the attack success of phytoseiid predators. Entomol Exp Appl 50:47–51
Blommers LHM, van Arendonk RCM (1979) The profit of senescence in phytoseiid mites. Oecologia 44:87–90
Bruce WA, Wrensch DL (1990) Reproductive potential, sex ratio, and mating efficiency of the straw itch mite (Acari: Pyemotidae). J Econ Entomol 83:384–391
Cadogan B, Laing J (1977) A technique for rearing the predaceous mite Balaustium putmani (Acarina: Erythraeidae), with notes on its biology and life history. Can Entomol 109:1535–1544
Carey JR (1993) Applied demography for biologists, with special emphasis on insects. Oxford University Press, New York, USA
Caswell H (1982) Life history theory and the equilibrium status of populations. Am Nat 120:317–339
Chang GC, Kareiva P (1999) The case for indigenous generalists in biological control. In: Hawkins BA, Cornell HV (eds) Theoretical approaches to biological control. Cambridge University Press, Cambridge, pp 103–115
Charnov EL, Schaffer WM (1973) Life-history consequences of natural selection: Cole’s result revisited. Am Nat 107:791–793
Childers CC, Rock GC (1981) Observations on the occurrence and feeding habits of Balaustium pulmani (Acari: Erythraeidae) in North Carolina apple orchards. Int J Acarol 7:63–68
Chiverton PA (1986) Predator density manipulation and its effects on populations of Rhopalosiphum padi (Hom, Aphididae) in spring barley. Ann Appl Biol 109:49–60
Cole LC (1954) The population consequences of life history phenomena. Q Rev Biol 29:103–137
Crawley MJ (2007) The R book. Wiley, Chichester
Diamond JM (1982) Big-bang reproduction and ageing in male marsupial mice. Nature 298:115–116
Faraji F, Janssen A, Sabelis MW (2002a) The benefits of clustering eggs: the role of egg predation and larval cannibalism in a predatory mite. Oecologia 131:20–26
Faraji F, Janssen A, Sabelis MW (2002b) Oviposition patterns in a predatory mite reduce the risk of egg predation caused by prey. Ecol Entomol 27:660–664
Getiva J, Acosta A (2004) Taxonomía de ácaros asociados a cultivos de flores. Asocolflores 66:59–68
Halliday R (2005) Predatory mites from crops and pastures in South Africa: potential natural enemies of redlegged earth mite Halotydeus destructor (Acari: Penthaleidae). Zootaxa 1079:11–64
Harwood JD, Phillips SW, Lello J, Sunderland KD, Glen DM, Bruford MW, Harper GL, Symondson WOC (2009) Invertebrate biodiversity affects predator fitness and hence potential to control pests in crops. Biol Contr 51:499–506
Hassell MP, May RM (1986) Generalist and specialist natural enemies in insect predator-prey interactions. J Anim Ecol 55:923–940
Hautekeete NC, Piquot Y, Van Dijk H (2001) Investment in survival and reproduction along a semelparity-iteroparity gradient in the Beta species complex. J Evol Biol 14:795–804
Hayes JL (1985) The predator-prey interaction of the mite Balaustium sp. and the pierid butterfly Colias alexandra. Ecology 66:300–303
Hedges BZ, Rosselot AE, Tomko PM, Yoder JA, Benoit JB (2012) High temperature and dehydration tolerance of the red velvet mite, Balaustium sp. (Erythraeidae), permit the exploitation of extremely hot, dry microhabitats. Int J Acarol 38:89–95
Hosmer DWJ, Lemeshow S (1999) Applied survival analysis. Regression modeling of time to event data. Wiley-Interscience Publication, New York
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biometrical J 50:346–363
Janssen A, Faraji F, van der Hammen T, Magalhães S, Sabelis MW (2002) Interspecific infanticide deters predators. Ecol Lett 5:490–494
Janssen A, Montserrat M, HilleRisLambers R, de Roos AM, Pallini A, Sabelis MW (2006) Intraguild predation usually does not disrupt biological control. In: Brodeur J, Boivin G (eds) Trophic and guild interactions in biological control, vol 3. Springer, Dordrecht, pp 21–44
Kaliszewski M, Athias-Binche F, Lindquist EE (1995) Parasitism and parasitoidism in Tarsonemina (Acari: Heterostigmata) and evolutionary considerations. Adv Parasitol 35:335–367
Magalhães S, Janssen A, Montserrat M, Sabelis MW (2005) Prey attack and predators defend: counterattacking prey trigger parental care in predators. Proc R Soc B 272:1929–1933
Makol J, Arijs Y, Wäckers FL (2012) A new species of Balaustium von Heyden, 1826 (Acari: Actinotrichida, Erythraeidae) from Spain. Zootaxa 3178:1–21
Messelink GJ, van Maanen R, van Steenpaal SEF, Janssen A (2008) Biological control of thrips and whiteflies by a shared predator: two pests are better than one. Biol Contr 44:372–379
Messelink G, van Maanen R, van Holstein-Saj R, Sabelis MW, Janssen A (2010) Pest species diversity enhances control of spider mites and whiteflies by a generalist phytoseiid predator. Biocontrol 55:387–398
Messelink GJ, Bloemhard CMJ, Cortes JA, Sabelis MW, Janssen A (2011) Hyperpredation by generalist predatory mites disrupts biological control of aphids by the aphidophagous gall midge Aphidoletes aphidimyza. Biol Contr 57:246–252
Messelink GJ, Sabelis MW, Janssen A (2012) Generalist predators, food web complexities and biological pest control in greenhouse crops. In: Larramendy ML, Soloneski S (eds) Integrated pest management and pest control—current and future tactics. InTech, Rijeka, Croatia, pp 191–214
Montserrat M, Bas C, Magalhaes S, Sabelis MW, de Roos AM, Janssen A (2007) Predators induce egg retention in prey. Oecologia 150:699–705
Muñoz K, Fuentes L, Cantor F, Rodríguez D, Cure JR (2009) Feeding preferences of the mite Balaustium sp. under controlled conditions. Agronomía Colombiana 27:95–103
Murdoch WW (1994) Population regulation in theory and practice. Ecology 75:271–287
Murdoch WW, Scott MA, Ebsworth P (1984) Effects of the general predator, Notonecta (Hemiptera) upon a fresh-water community. J Anim Ecol 53:791–808
Murdoch WW, Chesson J, Chesson PL (1985) Biological control in theory and practice. Am Nat 125:344–366
Nomikou M, Janssen A, Schraag R, Sabelis MW (2001) Phytoseiid predators as potential biological control agents for Bemisia tabaci. Exp Appl Acarol 25:271–291
Nomikou M, Janssen A, Schraag R, Sabelis MW (2002) Phytoseiid predators suppress populations of Bemisia tabaci on cucumber plants with alternative food. Exp Appl Acarol 27:57–68
Oelbermann K, Scheu S (2002) Effects of prey type and mixed dites on survival, growth and development of a generalist predator, Pardosa lugubris (Araneae : Lycosidae). Basic Appl Ecol 3:285–291
Pozzebon A, Duso C (2008) Grape downy mildew Plasmopara viticola, an alternative food for generalist predatory mites occurring in vineyards. Biol Contr 45:441–449
Putman WL (1969) Life history and behavior of Balaustium putmani (Acarina: Erythraeidae). Ann Entomol Soc Am 63:76–81
Ranta E, Tesar D, Kaitala V (2002) Environmental variability and semelparity vs. iteroparity as life histories. J Theor Biol 217:391–396
Roff DA (1992) The evolution of life histories: theory and analysis. Chapman & Hall, New York
Rosenheim JA (1998) Higher-order predators and the regulation of insect herbivore populations. Annu Rev Entomol 43:421–447
Rosenheim JA, Wilhoit LR, Armer CA (1993) Influence of intraguild predation among generalist insect predators on the suppression of an herbivore population. Oecologia 96:439–449
Rosenheim JA, Kaya HK, Ehler LE, Marois JJ, Jaffee BA (1995) Intraguild predation among biological control agents—theory and evidence. Biol Contr 5:303–335
Sabelis MW (1991) Life-history evolution of spider mites. In: Schuster R, Murphy PW (eds) The Acari. Reproduction, development and life-history strategies. Chapman & Hall, London, pp 23–49
Sabelis MW, Bruin J (1996) Evolutionary ecology: life history patterns, food plant choice and dispersal. In: Lindquist EE, Sabelis MW, Bruin J (eds) World crop pests, vol 6. Elsevier, Amsterdam, pp 329–366
Sabelis MW, Janssen A (1994) Evolution of life history patterns in the Phytoseiidae. In: Houck MA (ed) Mites: ecological and evolutionary analyses of life history patterns. Chapman & Hall, New York, USA, pp 70–98
Scheu S (2001) Plants and generalist predators as links between the below-ground and above-ground system. Basic Appl Ecol 2:3–13
Settle WH, Ariawan H, Astuti ET, Cahyana W, Hakim AL, Hindayana D, Lestari AS, Pajarningsih and Sartanto (1996) Managing tropical rice pests through conservation of generalist natural enemies and alternative prey. Ecology 77:1975–1988
Sonenshine DE (1991) Biology of ticks. Oxford University Press, New York
Stearns SC (1976) Life-history tactics: a review of the ideas. Q Rev Biol 51:3–47
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Symondson WOC, Sutherland KD, Greenstone MH (2002) Can generalist predators be effective biocontrol agents? Annu Rev Entomol 47:561–594
Toft S, Wise DH (1999) Growth, development, and survival of a generalist predator fed single- and mixed-species diets of different quality. Oecologia 119:191–197
Torrado E, Pérez M, Cure J, García M, Echeverri C (2001) Evaluación de sistemas de control biológico utilizados comercialmente en Europa para el control de plagas de rosa bajo invernadero, en la Sabana de Bogotá. Asocolflores 61:34–45
van Rijn PCJ, van Houten YM, Sabelis MW (2002) How plants benefit from providing food to predators even when it is also edible to herbivores. Ecology 83:2664–2679
Welbourn WC (1983) Potential use of trombidoid and erythraeoid mites as biological control agents of insect pests. In: Hoy MA, Cunningham GL, Knutson L (eds) Biological control of pests and mites. University of California, Berkeley, pp 103–140
Welbourn WC, Jennings DT (1991) Two new species of Erythraeidae (Acari, Prostigmata) associated with the spruce budworm, Choristoneura fumiferana (Clemens) (Lepidoptera, Tortricidae), in Maine. Can Entomol 123:567–580
Young TP (1981) A general model of comparative fecundity for semelparous and iteroparous life histories. Am Nat 118:27–36
Zeineddine M, Jansen VAA (2009) To age, to die: parity, evolutionary tracking and Cole’s paradox. Evolution 63:1498–1507
Acknowledgments
We thank Paul van Rijn for comments and especially for some pertinent suggestions with respect to Fig. 5, and two anonymous reviewers for comments. KM was supported by Colciencias (Colombia) (Programa “Francisco José de Caldas” 2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
The formal redescription of our Balaustium species is currently underway. It is going to be described as Balaustium leanderi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Muñoz-Cárdenas, K., Fuentes, L.S., Cantor, R.F. et al. Generalist red velvet mite predator (Balaustium sp.) performs better on a mixed diet. Exp Appl Acarol 62, 19–32 (2014). https://doi.org/10.1007/s10493-013-9727-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-013-9727-1