Abstract
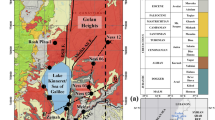
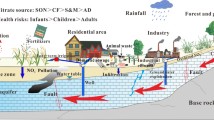
Hydrochemical and stable isotope (18O and 2H) analyses of groundwater samples were used to establish the hydrochemistry of groundwater in the Ankobra Basin. The groundwater was generally mildly acidic, low in conductivity and undersaturated with respect to carbonate phases. Major ions except bicarbonate were low and dissolved silica was moderately high. Silicate minerals weathering is probably the main process through which major ions enter the groundwater. Groundwater samples clustered tightly along the Global Meteoric Water Line suggesting integrative, smooth and rapid recharge from meteoric origin. The majority of the boreholes and a few hand dug wells cluster towards the Ca–Mg–HCO3 dominant section of the phase diagram, in conformity with the active recharge and short residence time shown by the isotope data. Aluminium, arsenic, manganese, iron and mercury were the only trace metals analysed with concentrations significantly above their respective detection limits. Approximately 20%, 5%, 40% and 25% respectively of boreholes had aluminium, arsenic, iron and manganese concentrations exceeding the respective WHO maximum acceptable limits for drinking water. The relatively large percentage of boreholes with high concentration of aluminium reflects the acidic nature of the groundwater.













Similar content being viewed by others
References
Acquah PC (1993) Emerging trends in gold ore processing and some related environmental issues in Ghana. Regional Trends in African Geology. Proc. of the 9th Int. Geol. Conf. (Accra, 2nd to 7th November 1992). Geological Society of Africa
Appiah H, Norman Di, Kuma JS, Nartey RS, Dankwa JBK (1993) Sources of diamonds in the Bonsa field. Regional Trends in African Geology. Proc. 9th Int. Geol. Conf. (Accra, 2nd to 7th November 1992). Geological Society of Africa
Appelo CAJ, Postma D (1999) Chemical analysis of groundwater. Geochemistry, Groundwater and Pollution. Fourth corrected print. AA Balkema, Rotterdam, Brookfield
Back W, Hanshaw B (1970) Comparison of chemical hydrology of the carbonate peninsulas of Florida and Yucatan. J Hydrol 10:330–368
Barcelona M, Gibb JP, Helfrich JA, Garske EE (1985) Practical Guide for Groundwater Sampling. Illinois State Water Survey ISWS Contract Report 374
Claasen HC (1982) Guidelines and techniques for obtaining water samples that accurately represent the water quality for an aquifer. U.S. Geological Survey Open File Report 82-1024, 49 pp
Caritat PDE (1995) Intensifying groundwater acidification at Birkenes, southern Norway. J Hydrol 170:47–67
Caritat PDE, Danilova S, Jaeger Ø, Reimann C, Storrø G (1998) Groundwater composition near the nickel-copper smelting industry on the Kola Peninsula, central Barents Region (NW Russia and NE Norway). J Hydrol 208:92–107
Carlos D, da Rosa JD, Lyon JS, Udall SL, Hocker PM (1997) Golden dreams, poisoned streams. Mineral Policy Center, Washington, DC
Craig H (1961) Isotopic variations in meteoric water. Science 133:1702–1703
Dickson KB, Benneh G (1980) A new geography of Ghana. Longmans Group Limited, London
Gale IN, Robins NS (1989) The Sampling and Monitoring of Groundwater Quality. British Geological Survey. Hydrogeology Report, No. 89/37
Garrels RM, Mackenzie FT (1967) Origin of the chemical compositions of some springs and lakes. In: Gound RF (ed) Equilibrium concepts in natural water systems. American Chemical Society Publications, Washington, DC
Hem JD (1985) Study and interpretation of chemical characteristics of natural water Geological Survey Water Supply Paper 2254. Washington, United States Government Printing Office, 264 p
Henriksen A, Kirkhusmo LA (1986) Water chemistry of acidified aquifers in southern Norway. Water Qual Bull 11(1):34–38, 61
Holden AV (1972) Present levels of mercury in man and his environment. In: Mercury contamination in man and his environment. Vienna, Internatioinal Atomic Energy Agency. Technical Report Series No. 137
Hounslow AW (1995) Water quality data analysis and interpretation. Lewis Publishers, Boca Raton, New York
Iaea (1983) Guidebook on nuclear techniques in hydrology (1983 edn). Technical reports series No. 91 IAEA, Vienna, 1983
Jacobson RL, Langmuir D (1970) The chemical history of some spring waters in carbonate rocks. Groundwater 8:5–9
Jankowski J, Acworth RI, Shekarforoush S (1998) Reverse ion-exchange in deeply weathered porphyritic dacite fractured aquifer system, Yass, New South Wales, Australia. In: Arehart GB, Hulston JR (eds) Proc. 9th Int. Symp. Water–Rock interaction. Taupo, New Zealand, 30 March–April 1998, 243–246. Balkema, Rotterdam
Junner NR, Hirst T, Service H (1942) Tarkwa Goldfield. Memoir, No. 6 Gold Coast Geological Survey
Kenoyer GJ, Bowser CJ (1992) Groundwater chemical evolution in a sandy silicate aquifer in Northern Wisconsin, 1. Patterns and Rates of Change. Water Resour Res 28(2):579–589
Kesse GO (1985) The mineral and rock resources of Ghana. AA Balkema, Rotterdam, Boston
Knutsson G (1994) Acidification effects on groundwater – prognosis of the risks for the future. In: Future groundwater resources at risk. Soveri J, Suokko T (eds) Proc. Helsinki Conf., June 1994. IAHS Publ. No. 222
Langmuir D (1971) The geochemistry of some carbonate groundwaters in central Pennsylvania. Geochim Cosmochim Acta 35:1023–1045
Langmuir D (1997) Aqueous environmental geochemistry. Prentice Hall, Upper Saddle River, New Jersey 07458
McLean W, Jankowski J (2000) Groundwater quality and sustainability in an alluvial aquifer, Australia. In: Sililo et al (eds) Proc. XXX IAH congress on Groundwater: Past Achievements and Future Challenges. Cape Town South Africa 26th November–1st December 2000. AA Balkema, Rotterdam, Brookfield
Nii Consult (1998) Information Building Block. Ghana Water management Study. Unpublished Consultancy Report for the Ministry of Works and Housing, Ghana, DANIDA, World Bank
Parkhurst DL and Appelo CAJ (1999) PHREEQC for windows version 1.4.07. A hydrogeochemical transport model. U.S. Geological Survey Software
Service (1938) Annual Report of the Gold-Coast Geological Survey
Smedley PL, Edmunds WM, West JM, Gardner SJ, Pelig-ba KB (1995) Vulnerability of Shallow Groundwater Quality due to Natural Geochemical Environment. Health Problems related to Groundwater in the Obuasi and Bolgatanga Areas, Ghana. Report prepared for ODA under the ODA/BGS Technology Development and Research Programme, Project 92/5
Stumm W (1992) Chemistry of the solid-water interface. John Wiley, New York
Tardy Y (1971) Characterization of the principal weathering types by the geochemistry of waters from some European and African crystalline massifs. Chem Geol 7:253–271
UNESCO/WHO/UNEP (1996) Water quality assessments. In: Chapman D (ed) A guide to the use of biota, sediments and water in environmental monitoring. E & FN SPON, London and New York
Von Brömssen U (1989) Acidification trends in Swedish groundwaters. Review of time series 1950–85. National Swedish Environmental Protection Board, Report 3547, 67 pp
White AF, Yee A (1985) Aqueous oxidation–reduction kinetics associated with coupled electron-cation transfer from iron-containing silicates at 25°C. Geochim Cosmochim Acta 49:1263–1275
World Health Organisation (WHO) (1980) Recommended health-based limits in occupational exposure to trace metals. Technical Report Series No. 647, Vienna
World Health Organisation (WHO) (1993) Guidelines for Drinking Water Quality. Revision of the 1984 Guidelines. Final Task Group Meeting. Geneva 21–25 September 1992
Acknowledgements
I wish also to express my gratitude to DANIDA, The Danish International Development Authority that partially funded the project through Grant No. Dau. 8l/300. I wish also to thank the Water Research Institute for providing the remainder of the funds for this project.
Author information
Authors and Affiliations
Corresponding author
Appendices
Rights and permissions
About this article
Cite this article
Kortatsi, B.K. Hydrochemical framework of groundwater in the Ankobra Basin, Ghana. Aquat Geochem 13, 41–74 (2007). https://doi.org/10.1007/s10498-006-9006-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-006-9006-4