Abstract
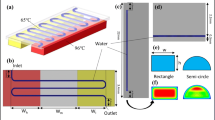
Continuous flow polymerase chain reactors (CFPCRs) are BioMEMS devices that offer unique capabilities for the ultra-fast amplification of target DNA fragments using repeated thermal cycling, typically over the following temperature ranges: 90°C–95°C for denaturation, 50°C–70°C for renaturation, and 70°C–75°C for extension. In CFPCR, DNA cocktail is pumped through the constant temperature zones and reaches thermal equilibrium with the channel walls quickly due to its low thermal capacitance. In previous work, a polycarbonate CFPCR was designed with microchannels 150 μm deep, 50 μm wide, and 1.78 m long—including preheating and post-heating zones, fabricated with LIGA, and demonstrated. The high thermal resistance of the polycarbonate led to a high temperature gradient in the micro-device at steady-state and was partly responsible for the low amplification yield. Several steps were taken to ensure that there were three discrete, uniform temperature zones on the polycarbonate CFPCR device including: reducing the thickness of the CFPCR substrate to decrease thermal capacitance, using copper plates as heating elements to ensure a uniform temperature input, and making grooves between temperature zones to increase the resistance to lateral heat conduction between zones. Finite element analyses (FEA) were used to evaluate the macro temperature distribution in the CFPCR device and the micro temperature distribution along a single microchannel. At steady-state, the simulated CFPCR device had three discrete temperature zones, each with a uniform temperature distribution with a variation of ±0.3°C. An infrared (IR) camera was used to measure the steady-state temperature distribution in the prototype CFPCR and validated the simulation results. The temperature distributions along a microchannel at flow velocities from 0 mm/s to 6 mm/s were used to estimate the resulting temperatures of the DNA reagents in a single microchannel. A 500 bp DNA fragment was generated from a bacteriophage λ-DNA target using 20 cycles of PCR. The amplification efficiencies compared to a commercial thermal cycler were 72.7% (2 mm/s), 44% (3 mm/s), and 29.4% (4 mm/s). The amplification efficiency with the modified CFPCR device increased by 363% at 2 mm/s and 440% at 3 mm/s compared to amplification obtained using a CFPCR device with the same fluidic layout, (Hashimoto et al., Lab Chip 4:638, 2004) strictly due to the improved temperature distribution.








Similar content being viewed by others
References
ANSYS CFD FLOTRAN Analysis Guide, SAS Ip, Inc., 3rd, (1997)
Y.D. Bejat, M.S. Thesis, Louisiana State University, Baton Rouge, LA, (2001).
Y. Bejat, X. Liu, D.E. Nikitopoulos, M.C. Murphy, M.W. Mitchell, S.A. Soper, A Continuous Flow Polymerization Chain Reaction (CFPCR) Micro-Chip, Bulletin of the American Physical Society, 55th Annual Meeting of the APS Division of Fluid Dynamics, Dallas TX, 47, 10, 15 (November 24–26, 2002)
T.D. Boone, Z.H. Fan, H.H. Hooper, A.J. Ricco, H. Tan, S.J. Williams, Anal. Chem. 74, 78A (Feb. 2002)
M. Bu, T. Melvin, G. Ensell, J.S. Wilkinson, A.G.R. Evans, J. Micromech. Microeng. 13, 125 (2003)
A.M. Chaudhari, T.M. Woudenberg, M. Albin, K.E. Goodson, JMEMS 7(4), 345 (1998)
J. Chen, M. Wabuyele, H. Chen, D. Patterson, M. Hupert, H. Shadpour, D.E. Nikitopoulos, S.A. Soper, Anal. Chem. 77, 658 (2005)
P.C. Chen, D.E. Nikitopoulos, S.A. Soper, M.C Murphy, InterPACK2007, Paper # IPACK2007-33330 (ASME, Vancouver, 2007)
J. Chiou, P. Matsudaira, A. Sonin, D. Enrlich, Anal. Chem. 73, 2018 (2001)
M. Curcio, J. Roeraade, Anal. Chem. 75(1), 1 (2003)
J.H. Daniel, S. Iqbal, R.B. Millington, D.F. Moore, C.R. Lowe, D.L. Leslie, M.A. Lee, M.J. Pearce, Sens. Actuators A 71, 81 (1998)
K.L. Davis, K.K.K. Liu, M. Lanan, M.D. Morris, Anal. Chem. 65, 293 (1993)
J. El-Ali, I.R. Perch-Nielsen, C.R. Poulsen, D.D. Bang, P. Telleman, A. Wolff, Sens. Actuators A 110, 3 (2004)
O. Geschke, H. Klank, P. Telleman, Microsystem Engineering of Lab-on-a-chip Devices (WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2004), Chap 5
B.C. Giordano, J. Ferrance, S. Swedberg, A.F.R. Huhmer, J.P. Landers, Anal. Biochem. 291, 124 (2001)
Q. Hao, J. Micromech. Microeng. 14, 914 (2004)
M. Hashimoto, P.C. Chen, M.W. Mitchell, D.E. Nikitopoulos, S.A. Soper, M.C Murphy, Lab Chip 4, 638 (2004)
A. Iles, R. Fortt, A.J. de Mello, Lab Chip 5, 540 (2005)
F.P. Incropera, D.P. Dewitt, Fundamentals of Heat and Mass Transfer, 5th edn. (Wiley, Danvers, 2001), Appendix
M.A. Innis, K.B. Myambo, D.H. Gelfand, M.A.D. Brow, PNAS. USA 85, 9436 (1988)
M.U. Kopp, A.J. DeMello, A. Manz, Science 280(5366), 1046 (1998)
K.K. Lee, K. Liu, K.L. Davis, M.D. Morris, Anal. Chem. 66, 3744 (1994)
S. Li, D.Y. Fozdar, M.F. Ali, H. Li, D. Shao, D.M. Vykoukal, J. Vykoukal, P.N. Floriano, M. Olsen, J.T. McDevitt, P.R.C. Gascoyne, S. Chen, JMEMS 15(1), 223 (2006)
C.S. Liao, G.B. Lee, J.J. Wu, C.C. Chang, T.M. Hsien, F.C. Huang, C.H. Luo, Biosens. Bioelectron. 20, 1341 (2005)
Y.C. Lin, C.C. Yang, M.Y. Huang, Sens. Actuators B 71, 127 (2000)
M.W. Mitchell, M.S. Thesis, LSU, December 2002.
K.B. Mullis, F. A. Faloona, Methods Enzymol. 155, 335 (1987)
H. Nagai, Y. Murakami, Y. Morita, K. Yokoyama, E. Tamiya, Anal. Chem. 73, 1043 (2001)
J. Noh, S.W. Sung, M.K. Jeon, S.H Kim, L.P. Lee, S.I. Woo, Sens. Actuators A 122, 196 (2005)
P.J. Obeid, T.K. Christopoulos, Anal. Chim. Acta 494, 1 (2003)
D. Ross, M. Gaitan, L.E. Locascio, Anal. Chem. 73, 4117 (2001)
D.J. Sadler, R. Changrani, P. Robers, C.F. Chou, F. Zenhausern, IEEE Trans. Compon. Packag. Technol. 26(2), 309 (2003)
S.D. Senturia, Microsystem Design (Springer Science + Business Media, New York, 2001), Chap. 22
Y.S. Shin, K. Cho, S.H. Lim, S. Chung, S.J. Park, C. Chung, D.C. Han, J.K. Chang, J. Micromech. Microeng. 13, 768 (2003)
S.A. Soper, S.M. Ford, S. Qi, R.L. McCarley, K. Kelly, M.C. Murphy, Anal. Chem. 643A (Oct. 2000)
S.C. Terry, J.H. Jerman, J.B. Angell, IEEE Trans. Electron Devices 26, 1880 (1979)
W. Wang, Z.X. Li, R. Luo, S.H. Lu, A.D. Xu, Y.J. Yang, J. Micromech. Microeng. 15, 1369 (2005)
D.S. Yoon, Y.S. Lee, Y. Lee, H.J. Cho, S.W. Sung; K.W. Oh, J. Cha, G. Lim, J. Micromech. Microeng. 12, 813 (2002)
M. Yuan, R. Pal, M.A. Burns, J. Micromech. Microeng. 15, 221 (2005)
Acknowledgements
The authors thank Dr. Makgorzata Witek from Center for BioModular Multi-Scale Systems for assisting with experimental questions, Mr. Jason Guy in the Center for BioModular Multi-Scale Systems for microfabrication, and also express gratitude to Dr. Srinath V. Ekkad and Mr. Yap-Sheng Goh for assistance with and use of the IR camera. This work was funded by a Bioengineering Research Partnership (NIH R24-EB002115) through the National Human Genome Research Institute (NHGRI), National Cancer Institute (NCI), the National Institute of BioImaging and Bioengineering (NIBIB) of the National Institutes of Health (NIH), the National Science Foundation under Grant Number EPS-0346411, and the State of Louisiana Board of Regents Support Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, PC., Nikitopoulos, D.E., Soper, S.A. et al. Temperature distribution effects on micro-CFPCR performance. Biomed Microdevices 10, 141–152 (2008). https://doi.org/10.1007/s10544-007-9119-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10544-007-9119-6