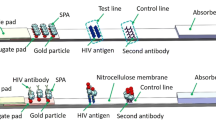
Abstract
The building blocks for an inexpensive, disposable, luminescence-based microfluidic immunoassay cassette are described, and their integration in a point-of-care diagnostic system is demonstrated. Fluid motion in the cassette is driven by depressing finger-actuated pouches. All reagents needed for the immunoassay can be stored in the cassette in liquid form. Prior to use, the cassette consists of two separate parts. A top storage component contains pouches, sealed storage chambers, a metering chamber, and needle seats. The bottom processing component contains connection needles, a mixing chamber, and a detection chamber with immobilized proteins. Subsequent to sample introduction, the storage and processing components are mated. The needles form hydraulic connections between the two parts and, in some cases, close valves. The pouches are then actuated sequentially to induce flow of various reagents and facilitate process operations. The cassette is compatible with different detection modalities. Both a cassette with immunochromatographic-based detection and a cassette with microbead-based detection were constructed and evaluated. The immunochromatographic cassette was used to detect antibodies to HIV in saliva samples. The bead-based cassette was used to detect the proinflammatory chemokine IL-8. The experimental data demonstrates good repeatability and reasonable sensitivity.











Similar content being viewed by others
References
R.C. Anderson, X. Su, G.J. Bogdan, J. Fenton, A miniature integrated device for automated multistep genetic assays. Nucleic Acids Res. 28, i–vi (2000)
K.D. Barbee, X. Huang, Magnetic assembly of high-density DNA arrays for genomic analyses. Anal. Chem. 80, 2149–2154 (2008)
H.H. Bau, J. Zhong, M. Yi, A minute magneto hydro dynamic (MHD) mixer. Sens. Actuators B 79, 207–215 (2001)
A. Bhattacharyya, C.M. Klapperich, Design and testing of a disposable microfluidic chemiluminescent immunoassay for disease biomarkers in human serum samples. Biomedical Microdevices 9, 245–251 (2007)
T.M. Blicharz, W.L. Siqueira, E.J. Helmerhorst, F.G. Oppenheim, P.J. Wexler, F.F. Little, D.R. Walt, Fiber-optic microsphere-based antibody array for the analysis of inflammatory cytokines in saliva. Anal. Chem. 81, 2106–2114 (2009)
D.L.N. Cardy, G.J. Allen, Lateral flow assay device and method, International Patent, Publication No.: WO/2004/007078
M.M. Caulum, C.S. Henry, Multi-analyte immunoassay using cleavable tags and microchip micellular electrokinetic chromatography. Analyst 131, 1091–1093 (2006)
Z. Chen, M.G. Mauk, J. Wang, W.R. Abrams, P.L.A.M. Corstjens, R.S. Niedbala, D. Malamud, H.H. Bau, A microfluidic system for saliva-based detection of infectious diseases, Ann. N.Y. Acad. Sci. 1098, 429-436 (2007)
P.L.A.M. Corstjens, Z. Chen, M. Zuiderwijk, H.H. Bau, W.R. Abrams, D. Malamud, R.S. Niedbala, H.J. Tanke, Rapid assay format for multiplex detection of humoral immune responses to infectious disease pathogens (HIV, HCV, and TB). Ann. N.Y. Acad. Sci 1098, 437–445 (2007)
P.L.A.M. Corstjens, M. Zuiderwijk, H.J. Tanke, J. van der Ploeg-Schip, T.H.M. Ottenhoff, A. Geluk, A user-friendly, highly sensitive assay to detect the IFN-γ secretion by T cells. Clin. Biochem. 6, 440–444 (2008)
J.B. Fan, K.L. Gunderson, M. Bibikova, J.M. Yeakley, J. Chen, E. Wickham Garcia, L.L. Lebruska, M. Laurent, R. Shen, D. Barker, Illumina universal bead arrays. Methods Enzymol. 410, 57–73 (2006)
J.A. Ferguson, F.J. Steemers, D.R. Walt, High-density fiber-optic DNA random microsphere array. Anal. Chem. 72, 5618–5624 (2000)
H.J.G.E. Gardeniers, R. Luttge, E.J.W. Berenschot, M.J. de Boer, S.Y. Yeshurun, M. Hefetz, R. van’t Oever, A. van den Berg, Silicon micromachined hollow microneedles for transdermal liquid transport. Journal of Microelectromechanical Systems 6, 855–862 (2003)
A. Goodey, J.J. Lavigne, S.M. Savoy, M.D. Rodriguez, T. Curey, A. Tsao, G. Simmons, J. Wright, S. Yoo, Y. Sohn, E.V. Anslyn, J.B. Shear, D.P. Neikirk, J.T. McDevitt, Development of multianalyte sensor arrays composed of chemically derivatized polymeric microspheres localized in micromachined cavities. J. Am. Chem. Soc. 123, 2559–2570 (2001)
W. Gu, X. Zhu, N. Futai, B.S. Cho, S. Takayama, Computerized microfluidic cell culture using elastomeric channels and Braille displays. PNAS 45, 15861–15866 (2004)
G.G. Guilbault, Practical fluorescence, 2nd edition, CRC Press (ISBN 0824783506, 9780824783501) (1990)
S. Haeberle, R. Zengerle, Microfluidic platforms for lab-on-a-chip applications. Lab Chip 7, 1094–1110 (2007)
J. Hampl, M. Hall, N.A. Mufti, Y.M. Yao, D.B. MacQueen, W.H. Wright, D.E. Cooper, Upconverting phosphor reporters in immunochromatographic assays. Anal. Biochem. 288, 176–187 (2001)
M. Hashimoto, F. Barany, S.A. Soper, Polymerase chain reaction/ligase detection reaction/hybridization assays using flow-through microfluidic devices for the detection of low-abundant DNA point mutations. Biosens. Bioelectron. 10, 1915–1923 (2006)
W.Z. Ho, Self-contained microfluidic biochip and apparatus, United States Patent, Patent No.: US 7122153 (2006)
K. Kim, J.B. Lee, High aspect ratio tapered hollow metallic microneedle arrays with microfluidic interconnector. Microsyst. Technol. 13, 231–235 (2007)
D.S. Kim, S.H. Lee, C.H. Ahn, J.Y. Lee, T.H. Kwon, Disposable integrated microfluidic biochip for blood typing by plastic microinjection molding. Lab Chip 6, 794–802 (2006)
C.Y. Lee, G.B. Lee, J.L. Lin, F.C. Huang, C.S. Liao, Integrated microfluidic systems for cell lysis, mixing/pumping and DNA amplification. J. Micromechanics Microengineering 15, 1215–1223 (2005)
S. Li, P.N. Floriano, N. Christodoulides, D.Y. Fozdar, D. Shao, M.F. Ali, P. Dharshan, S. Mohanty, D. Neikirk, J.T. McDevitt, S. Chen, Disposable polydimethylsiloxane/silicon hybrid chips for protein detection. Biosens. Bioelectron 21, 574–580 (2005)
C.T. Lim, Y. Zhang, Bead-based microfluidic immunoassays: the next generation. Biosens. Bioelectron. 22, 1197–1204 (2007)
C. Liu, X. Qiu, S. Ongagna, D. Chen, Z. Chen, W.R. Abrams, P.L. Corstjens, H.H. Bau, A timer-actuated immunoassay cassette for detecting molecular markers in oral fluids. Lab Chip 9, 768–776 (2009)
D. Malamud, H. Bau, S. Niedbala, P. Corstjens, Point detection of pathogens in oral samples. Adv. Dent. Res. 18, 12–16 (2005)
M.G. Mauk, B.L. Ziober, Z. Chen, J.A. Thompson, H.H. Bau, Lab-on-a-chip technologies for oral-based cancer screening and diagnostics: capabilities, issues, and prospects. Ann. N.Y. Acad. Sci. 1098, 467–475 (2007)
J.K. Ng, E.S. Selamat, W. Liu, A spatially addressable bead-based biosensor for simple and rapid DNA detection. Biosens. Bioelectron. 23, 803–810 (2008)
S.J. Paik, S. Byun, J.M. Lim, Y. Park, A. Lee, S. Chung, J. Chang, K. Chun, D. Cho, In-plane single-crystal-silicon microneedles for minimally invasive microfluid systems. Sens. Actuators A 114, 276–284 (2004)
P.M. Pilarski, S. Adamia, C.J. Backhouse, An adaptable microvalving system for on-chip polymerase chain reactions. J. Immunol. Methods 305, 48–58 (2005)
S. Qian, H.H. Bau, A mathematical model of lateral flow bio-reactions applied to sandwich assays. Anal. Biochem. 322, 89–98 (2003)
S. Qian, H.H. Bau, Analysis of lateral flow bio-detectors: competitive format. Anal. Biochem. 326, 211–224 (2004)
S. Ramachandran, J. Gerdes, P. Tarr, P. Yager, L. Dillman, R. Peck, M. Kokoris, M. Nabavi, F. Battrell, D. Hoekstra, B.H. Weigl, Dry-reagent storage for disposable lab-on-a-card diagnosis of enteric pathogens, Proceedings of the 1st Distributed Diagnosis and Home Healthcare (D2H2) Conference Arlington, 16–19 (2006)
D. Snakenborg, G. Perozziello, O. Geschke, J.P. Kutter, A fast and reliable way to establish fluidic connections to planar microchips. J. Micromechanics Microengineering 17, 98–103 (2007)
F. van de Rijke, H. Zijlmans, S. Li, T. Vail, A.K. Raap, R.S. Niedbala, H.J. Tanke, Up-converting phosphor reporters for nucleic acid microarrays. Nat. Biotechnol. 19, 273–276 (2001)
J. Wang, Z. Chen, P.L.A.M. Corstjens, M.G. Mauk, H.H. Bau, A disposable microfluidic cassette for DNA amplification and detection. Lab Chip 6, 46–53 (2006)
R. Wilkinson, D. Rowland, W.M. Ching, Development of an improved rapid lateral flow assay for the detection of orientia tsutsugamushi-specific IgG/IgM antibodies. Ann. N.Y. Acad. Sci. 990, 386–390 (2003)
W. Xu, K. Sur, H. Zeng, A. Feinerman, D. Kelso, J.B. Ketterson, A microfluidic approach to assembling ordered microsphere arrays. J. Micromechanics Microengineering 18, 1–6 (2008)
M. Yi, H.H. Bau, The kinematics of bend-induced mixing in micro-conduits. Int. J. Heat Fluid Flow 24, 645–656 (2003)
C. Zhang, D. Xing, Y. Li, Micropumps, microvalves, and micromixers within PCR microfluidic chips: advances and trends. Biotechnol. Adv. 25, 483–514 (2007)
Acknowledgments
The research was funded, in part, by National Institute of Health Grants U01-DE-017855 and U01-DE-017788, and National Science Foundation Integrative Graduate Education and Research Traineeship Program Grant DGE-0221664 to JAT. The samples used in this study were collected by the Women's Interagency HIV Study and its associated Collaborative Study Groups. Dr. David Walt's group at Tufts University provided the Illumina chiplet and the reagents and protocols to carry out the microbead array tests. Dr. Timothy Blicharz (a graduate of Walt's group) assisted with the microbead detection assay. Ms. Mian Qin provided insights into the mixing process in the cassette.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qiu, X., Thompson, J.A., Chen, Z. et al. Finger-actuated, self-contained immunoassay cassettes. Biomed Microdevices 11, 1175–1186 (2009). https://doi.org/10.1007/s10544-009-9334-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10544-009-9334-4