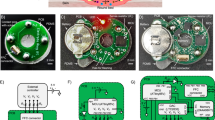
Abstract
We present the first implantable drug delivery system for controlled timing and location of dosing in small animals. Current implantable drug delivery devices do not provide control over these factors nor are they feasible for implantation in research animals as small as mice. Our system utilizes an integrated electrolysis micropump, is refillable, has an inert drug reservoir for broad drug compatibility, and is capable of adjustment to the delivery regimen while implanted. Electrochemical impedance spectroscopy (EIS) was used for characterization of electrodes on glass substrate and a flexible Parylene substrate. Benchtop testing of the electrolysis actuator resulted in flow rates from 1 μL/min to 34 μL/min on glass substrate and up to 6.8 μL/min on Parylene substrate. The fully integrated system generated a flow rate of 4.72 ± 0.35 μL/min under applied constant current of 1.0 mA while maintaining a power consumption of only ~3 mW. Finally, we demonstrated in vivo application of the system for anti-cancer drug delivery in mice.













Similar content being viewed by others
References
Alzet. ALZET® Osmotic Pumps—Implantable pumps for research. Available at: http://www.alzet.com/ [Accessed October 19, 2011a]
J. Ambati et al., Transscleral delivery of bioactive protein to the choroid and retina. Investig Ophthalmol Vis Sci 41(5), 1186–1191 (2000)
F. Amirouche, Y. Zhou, T. Johnson, Current micropump technologies and their biomedical applications. Microsyst Technol 15, 647–666 (2009)
A. Bard, Electrochemical methods: fundamentals and applications, 2nd edn. (Wiley, New York, 2001)
C. Belmont, H.H. Girault, Coplanar interdigitated band electrodes for synthesis Part I: ohmic loss evaluation. J Appl Electrochem 24, 475–480 (1994)
E. Benedikz, E. Kloskowska, B. Winblad, The rat as an animal model of Alzheimer’s disease. J Cell Mol Med 13, 1034–1042 (2009)
S. Böhm, W. Olthuis, P. Bergveld, An integrated micromachined electrochemical pump and dosing system. Biomed Microdevices 1(2), 121–130 (1999)
Debiotech. Debiotech—Medical Devices—Switzerland. Available at: http://www.debiotech.com/ [Accessed November 18, 2011b]
D.M. Dykxhoorn, D. Palliser, J. Lieberman, The silent treatment: siRNAs as small molecule drugs. Gene Ther 13(6), 541–552 (2006)
N. Elman et al., The next generation of drug-delivery microdevices. Clin Pharmacol Ther 85(5), 544–547 (2009)
A.T. Evans, S. Chiravuri, Y.B. Gianchandani, A multidrug delivery system using a piezoelectrically actuated silicon valve manifold with embedded sensors. J Microelectromechanical Syst 20, 231–238 (2011)
O.C. Farokhzad, R. Langer, Impact of nanotechnology on drug delivery. ACS Nano 3, 16–20 (2009)
F. Faupel, R. Willecke, A. Thran, Diffusion of metals in polymers. Mater Sci Eng R Rep 22(1), 1–55 (1998)
H. Gensler et al., Implantable MEMS drug delivery device for cancer radiation reduction. In Proc. IEEE Int. Conf. MEMS, (IEEE Int. Conf. MEMS, Hong Kong, SAR, China, 2010), pp. 23–26
H. Gensler, R. Sheybani, E. Meng, Rapid non-lithography based fabrication process and characterization of Parylene C bellows for applications in MEMS electrochemical actuators. In Proc. IEEE Int. Conf. TRANSDUCERS, (IEEE Int. Conf. TRANSDUCERS, Beijing, China, 2011), pp. 2347–2350
A.C.R. Grayson et al., Multi-pulse drug delivery from a resorbable polymeric microchip device. Nat Mater 2, 767–772 (2003)
iPrecio. iPRECIO®, Innovative Drug Infusion Technology for Laboratory Animals. Available at: http://www.iprecio.com/ [Accessed October 19, 2011c]
J.W., Judy, T. Tamagawa, D.L., Polla, Surface-machined micromechanical membrane pump. In Proc. IEEE Int. Conf. MEMS IEEE Int. Conf. MEMS, (IEEE, Nara, Japan, 1991), pp. 182–186
I.O. K’Owino, O.A. Sadik, Impedance spectroscopy: a powerful tool for rapid biomolecular screening and cell culture monitoring. Electroanalysis 17, 2101–2113 (2005)
K. Kramer et al., The use of radiotelemetry in small laboratory animals: recent advances. Contemp Topics Lab Anim Sci / Am Assoc Lab Anim Sci 40(1), 8–16 (2001)
D.J. Laser, J.G. Santiago, A review of micropumps. J Micromech Microeng 14, R35–R64 (2004)
L.-H. Lee, Fundamentals of adhesion (Plenum Press, New York, 1991)
J.H. Lee et al., Microstructure and adhesion of Au deposited on Parylene-c substrate with surface modification for potential immunoassay application. IEEE Trans Plasma Sci 32, 505–509 (2004)
P.-Y. Li, Implantable bioMEMS drug delivery systems. Ph.D. Dissertation (Dept. Biomed. Eng., University of Southern California, Los Angeles, 2009)
Y. Li et al., In vivo release from a drug delivery MEMS device. J Control Release 100(2), 211–219 (2004)
P.-Y. Li et al., An electrochemical intraocular drug delivery device. Sens Actuators A Phys 143(1), 41–48 (2008)
P.-Y. Li, et al., A Parylene bellows electrochemical actuator for intraocular drug delivery, in Proc. IEEE Int. Conf. TRANSDUCERS, (IEEE Int. Conf. TRANSDUCERS, Denver, CO, USA, 2009), pp. 1461–1464
P.-Y. Li et al., A Parylene bellows electrochemical actuator. J Microelectromechanical Syst 19(1), 215–228 (2010)
R. Lo, E. Meng, A modular heat-shrink-packaged check valve with high pressure shutoff. J Microelectromechanical Syst 20, 1163–1173 (2011)
R. Lo, E. Meng, In-plane Bandpass Regulation Check Valve in Heat-Shrink Packaging for Drug Delivery. In Proc. IEEE Int. Conf. MEMS. (IEEE Int. Conf. MEMS, Sorrento, Italy, 2009), pp. 236–239
R. Lo et al., A passive MEMS drug delivery pump for treatment of ocular diseases. Biomed Microdevices 11, 959–970 (2009)
D. Maillefer et al., A high-performance silicon micropump for an implantable drug delivery system, in Proc. IEEE Int. Conf. MEMS. IEEE Int. Conf. MEMS, (IEEE, Orlando, FL, USA, 1999), pp. 541–546
D. Maillefer et al., A high-performance silicon micropump for disposable drug delivery systems, in Proc. IEEE Int. Conf. MEMS. IEEE Int. Conf. MEMS. Interlaken, (IEEE, Switzerland, 2001), pp. 413–417
Med-e-Cell. Welcome to Med-e-Cell: infusion pumps, fluid delivery devices, oxygen generation/control and fuel cell technology for licensing and development partnerships. Available at: http://medecell.com/ [Accessed October 19, 2011d]
E. Meng, P.-Y. Li, Y.-C. Tai, A biocompatible Parylene thermal flow sensing array. Sens Actuators A Phys 144, 18–28 (2008)
C.R. Neagu, A Medical Microactuator based on an Electrochemical Principle (University of Twente, Entschede, 1998)
C.R. Neagu, The electrolysis of water: an actuation principle for MEMS with a big opportunity. Mechatronics 10, 571–581 (2000)
C.R. Neagu et al., An electrochemical microactuator: principle and first results. J Microelectromechanical Syst 5, 2–9 (1996)
N.T. Nguyen, X. Huang, T.K. Chuan, MEMS-micropumps: a review. J Fluids Eng 124(2), 384–392 (2002)
A. Parthasarathy, The platinum microelectrode/Nafion interface: an electrochemical impedance spectroscopic analysis of oxygen reduction kinetics and Nafion characteristics. J Electrochem Soc 139, 1634 (1992)
S. Petit-Zeman, Rat genome sequence reignites preclinical model debate. Nat Rev Drug Discov 3, 287–288 (2004)
X. Ruan, Drug-related side effects of long-term intrathecal morphine therapy. Pain Physician 10(2), 357–366 (2007)
J.T. Santini Jr. et al., Microchips as controlled drug-delivery devices. Angew Chem Int Ed 39, 2396–2407 (2000)
D. Sawyer, Experimental electrochemistry for chemists (Wiley, New York, 1974)
R. Sheybani, E. Meng, High efficiency wireless electrochemical actuators: Design, fabrication and characterization by electrochemical impedance spectroscopy, in Proc. IEEE Int. Conf. MEMS. (IEEE Int. Conf. MEMS, Cancun, Mexico, 2011), pp. 1233–1236
R. Sheybani, H. Gensler, E. Meng, Rapid and repeated bolus drug delivery enabled by high efficiency electrochemical bellows actuators, in Proc. IEEE Int. Conf. TRANSDUCERS. IEEE Int. Conf. TRANSDUCERS, (IEEE, Beijing, China, 2011), pp. 490493
J.G. Smits, Piezoelectric micropump with microvalves, in Proc. University/Government/Industry Microelectronics Symposium. University/Government/Industry Microelectronics Symposium, (IEEE 1989), pp. 92–94
M.H. Smolensky, N.A. Peppas, Chronobiology, drug delivery, and chronotherapeutics. Adv Drug Deliv Rev 59(9–10), 828–851 (2007)
M. Staples, Microchips and controlled-release drug reservoirs. Wiley Interdiscip Rev Nanomedicine Nanobiotechnology 2, 400–417 (2010)
F.L. Trull, B.A. Rich, More regulation of rodents. Science 284, 1463 (1999)
N.C. Tsai, C.Y. Sue, Review of MEMS-based drug delivery and dosing systems. Sens Actuators A Phys 134(2), 555–564 (2007)
J. Urquhart, J.W. Fara, K.L. Willis, Rate-controlled delivery systems in drug and hormone research. Annu Rev Pharmacol Toxicol 24, 199–236 (1984)
A.V. Vasenkov, Atomistic modeling of Parylene-metal interactions for surface micro-structuring. J Mol Model 17(12), 3219–3228 (2011)
P. Woias, Micropumps–past, progress and future prospects. Sens Actuators B Chem 105(1), 28–38 (2005)
K.C. Worley, G.M. Weinstock, R.A. Gibbs, Rats in the genomic era. Physiol Genomics 32, 273–282 (2007)
P.K. Wu et al., Metal/polymer interface adhesion by partially ionized beam deposition. J Appl Phys 80, 5759 (1996)
B.B. Youan, Chronopharmaceutics: gimmick or clinically relevant approach to drug delivery? J Control Release 98(3), 337–353 (2004)
K.-S. Yun, E. Yoon, Micropumps for MEMS/NEMS and Microfluidic Systems, in MEMS/NEMS, ed. by C.T. Leondes (Springer US, Boston, 2006), pp. 1112–1144
R. Zengerle, A. Richter, H. Sandmaier, A micro membrane pump with electrostatic actuation, in Proc. IEEE Int. Conf. MEMS. IEEE Int. Conf. MEMS. Travemunde, (IEEE, Germany, 1992), pp. 19–24
Acknowledgements
This work was supported in part by the Wallace H. Coulter Foundation Early Career Translational Research Award, National Institutes of Health/National Eye Institute under award number R21EY018490, and National Institutes of Health/National Institute on Drug Abuse under award number R21DA026970. H.G. was supported by a National Science Foundation Graduate Research Fellowship. The authors would like to thank Dr. Ken-Tye Yong, Dr. Indrajit Roy and Dr. Paras N. Prasad of University of Buffalo, The State University of New York, for providing the gold nanoparticles; Dr. Rizwan Masood and Dr. Uttam K. Sinha for providing the HNB-001 siRNA-gold nanoplexes and their surgical expertise; and Sutao Zhu of the University of Southern California for her surgical expertise. We would also like to thank Dr. Donghai Zhu and the members of the USC Biomedical Microsystems Laboratory for their assistance with this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gensler, H., Sheybani, R., Li, PY. et al. An implantable MEMS micropump system for drug delivery in small animals. Biomed Microdevices 14, 483–496 (2012). https://doi.org/10.1007/s10544-011-9625-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10544-011-9625-4