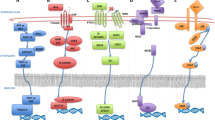
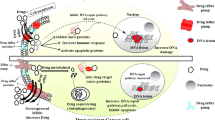
Abstract
Therapeutic resistance has been and remains to be the major challenge in developing successful treatments for different cancers and therefore, understanding the underlying mechanisms in the development of therapeutic resistance is crucial in combating cancers. Multiple mechanisms underlie the development of therapeutic resistance, and the signaling pathways involved in cancer stem cell repopulation, enhanced epithelial-mesenchymal transition (EMT), inflammatory infiltration, and immunosuppression play pivotal roles in this process. Accumulating evidence indicates that the COX2/PGE2/EP axis plays crucial roles not only in tumor development including initiation and progression but also in the development of therapeutic resistance. In this review, we will first dissect the relationship between the COX2/PGE2/EP axis and therapeutic resistance by focusing on the roles of the COX2/PGE2/EP axis in cancer stem cell repopulation, EMT, and anti-cancer immunity. Then, we will summarize the currently available compounds/drugs targeting each component of this axis as well as some of the underlying mechanisms. We hope that better understanding the underlying mechanisms of the functional compounds will be helpful in seeking additive and/or synergistic effects against therapeutic resistance without or with minimal adverse consequence.





Similar content being viewed by others
References
Siegel, R. L., Miller, K. D., & Jemal, A. (2017). Cancer statistics, 2017. CA: a Cancer Journal for Clinicians, 67(1), 7–30. https://doi.org/10.3322/caac.21387.
Lou, F., Huang, J., Sima, C. S., Dycoco, J., Rusch, V., & Bach, P. B. (2013). Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. The Journal of Thoracic and Cardiovascular Surgery, 145(1), 75–81; discussion 81-72. https://doi.org/10.1016/j.jtcvs.2012.09.030.
Mantovani, A., Allavena, P., Sica, A., & Balkwill, F. (2008). Cancer-related inflammation. Nature, 454(7203), 436–444. https://doi.org/10.1038/nature07205.
Chan, A. T., Ogino, S., & Fuchs, C. S. (2009). Aspirin use and survival after diagnosis of colorectal cancer. JAMA, 302(6), 649–658. https://doi.org/10.1001/jama.2009.1112.
Wu, W. K., Sung, J. J., Lee, C. W., Yu, J., & Cho, C. H. (2010). Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers: an update on the molecular mechanisms. Cancer Letters, 295(1), 7–16. https://doi.org/10.1016/j.canlet.2010.03.015.
Wang, D., & Dubois, R. N. (2010). Eicosanoids and cancer. Nature Reviews. Cancer, 10(3), 181–193. https://doi.org/10.1038/nrc2809.
Chen, E. P., & Smyth, E. M. (2011). COX-2 and PGE2-dependent immunomodulation in breast cancer. Prostaglandins & Other Lipid Mediators, 96(1–4), 14–20. https://doi.org/10.1016/j.prostaglandins.2011.08.005.
Steinecker-Frohnwieser, B., Kaltenegger, H., Weigl, L., Mann, A., Kullich, W., Leithner, A., & Lohberger, B. (2017). Pharmacological treatment with diacerein combined with mechanical stimulation affects the expression of growth factors in human chondrocytes. Biochemistry and Biophysics Reports, 11, 154–160. https://doi.org/10.1016/j.bbrep.2017.06.006.
Peng, Y. J., Shen, T. L., Chen, Y. S., Mersmann, H. J., Liu, B. H., & Ding, S. T. (2018). Adiponectin and adiponectin receptor 1 overexpression enhance inflammatory bowel disease. Journal of Biomedical Science, 25(1), 24. https://doi.org/10.1186/s12929-018-0419-3.
Kim, H. S., Moon, H. G., Han, W., Yom, C. K., Kim, W. H., Kim, J. H., & Noh, D. Y. (2012). COX2 overexpression is a prognostic marker for stage III breast cancer. Breast Cancer Research and Treatment, 132(1), 51–59. https://doi.org/10.1007/s10549-011-1521-3.
(2005). PGE2 produced by lung cancer suppresses immune function through T-regulatory cells and can be blocked by the COX2 inhibitor celebrex. Cancer Biology & Therapy, 4(8), v–vi.
Banu, N., Buda, A., Chell, S., Elder, D., Moorghen, M., Paraskeva, C., Qualtrough, D., & Pignatelli, M. (2007). Inhibition of COX-2 with NS-398 decreases colon cancer cell motility through blocking epidermal growth factor receptor transactivation: possibilities for combination therapy. Cell Proliferation, 40(5), 768–779. https://doi.org/10.1111/j.1365-2184.2007.00459.x.
Cook, P. J., Thomas, R., Kingsley, P. J., Shimizu, F., Montrose, D. C., Marnett, L. J., Tabar, V. S., Dannenberg, A. J., & Benezra, R. (2016). Cox-2-derived PGE2 induces Id1-dependent radiation resistance and self-renewal in experimental glioblastoma. Neuro-Oncology, 18(10), 1379–1389. https://doi.org/10.1093/neuonc/now049.
Tong, D., Liu, Q., Liu, G., Xu, J., Lan, W., Jiang, Y., Xiao, H., Zhang, D., & Jiang, J. (2017). Metformin inhibits castration-induced EMT in prostate cancer by repressing COX2/PGE2/STAT3 axis. Cancer Letters, 389, 23–32. https://doi.org/10.1016/j.canlet.2016.12.031.
Liu, Q., Yuan, W., Tong, D., Liu, G., Lan, W., Zhang, D., et al. (2016). Metformin represses bladder cancer progression by inhibiting stem cell repopulation via COX2/PGE2/STAT3 axis. Oncotarget, 7(19), 28235–28246. https://doi.org/10.18632/oncotarget.8595.
Ko, C. J., Lan, S. W., Lu, Y. C., Cheng, T. S., Lai, P. F., Tsai, C. H., Hsu, T. W., Lin, H. Y., Shyu, H. Y., Wu, S. R., Lin, H. H., Hsiao, P. W., Chen, C. H., Huang, H. P., & Lee, M. S. (2017). Inhibition of cyclooxygenase-2-mediated matriptase activation contributes to the suppression of prostate cancer cell motility and metastasis. Oncogene, 36(32), 4597–4609. https://doi.org/10.1038/onc.2017.82.
Harris, R. E., Beebe-Donk, J., & Alshafie, G. A. (2008). Similar reductions in the risk of human colon cancer by selective and nonselective cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer, 8, 237. https://doi.org/10.1186/1471-2407-8-237.
Jia, H., Wang, H., Yao, Y., Wang, C., & Li, P. (2018). MiR-136 inhibits malignant progression of hepatocellular carcinoma cells by targeting cyclooxygenase 2. Oncology Research, 26, 967–976. https://doi.org/10.3727/096504018X15148192843443.
Teng, G., Dai, Y., Chu, Y., Li, J., Zhang, H., Wu, T., Shuai, X., & Wang, W. (2018). Helicobacter pylori induces caudal-type homeobox protein 2 and cyclooxygenase 2 expression by modulating microRNAs in esophageal epithelial cells. Cancer Science, 109(2), 297–307. https://doi.org/10.1111/cas.13462.
Codocedo, J. F., & Inestrosa, N. C. (2016). Wnt-5a-regulated miR-101b controls COX2 expression in hippocampal neurons. Biological Research, 49, 9. https://doi.org/10.1186/s40659-016-0071-x.
Hu, E., Ding, L., Miao, H., Liu, F., Liu, D., Dou, H., et al. (2015). MiR-30a attenuates immunosuppressive functions of IL-1beta-elicited mesenchymal stem cells via targeting TAB3. FEBS Letters, 589(24 Pt B), 3899–3907. https://doi.org/10.1016/j.febslet.2015.11.001.
Lai, Y., Zhang, X., Zhang, Z., Shu, Y., Luo, X., Yang, Y., et al. (2013). The microRNA-27a: ZBTB10-specificity protein pathway is involved in follicle stimulating hormone-induced VEGF, Cox2 and survivin expression in ovarian epithelial cancer cells. International Journal of Oncology, 42(2), 776–784. https://doi.org/10.3892/ijo.2012.1743.
He, Q., Chen, Z., Dong, Q., Zhang, L., Chen, D., Patel, A., Koya, A., Luan, X., Cabay, R. J., Dai, Y., Wang, A., & Zhou, X. (2016). MicroRNA-21 regulates prostaglandin E2 signaling pathway by targeting 15-hydroxyprostaglandin dehydrogenase in tongue squamous cell carcinoma. BMC Cancer, 16(1), 685. https://doi.org/10.1186/s12885-016-2716-0.
Lai, Y. H., Liu, H., Chiang, W. F., Chen, T. W., Chu, L. J., Yu, J. S., Chen, S. J., Chen, H. C., & Tan, B. C. M. (2018). MiR-31-5p-ACOX1 axis enhances tumorigenic fitness in oral squamous cell carcinoma via the promigratory prostaglandin E2. Theranostics, 8(2), 486–504. https://doi.org/10.7150/thno.22059.
Mehdawi, L. M., Satapathy, S. R., Gustafsson, A., Lundholm, K., Alvarado-Kristensson, M., & Sjolander, A. (2017). A potential anti-tumor effect of leukotriene C4 through the induction of 15-hydroxyprostaglandin dehydrogenase expression in colon cancer cells. Oncotarget, 8(21), 35033–35047. https://doi.org/10.18632/oncotarget.16591.
Akasaka, H., & Ruan, K. H. (2016). Identification of the two-phase mechanism of arachidonic acid regulating inflammatory prostaglandin E2 biosynthesis by targeting COX-2 and mPGES-1. Archives of Biochemistry and Biophysics, 603, 29–37. https://doi.org/10.1016/j.abb.2016.04.011.
Zhao, J., Wen, S., Wang, X., & Zhang, Z. (2017). Helicobacter pylori modulates cyclooxygenase-2 and 15-hydroxy prostaglandin dehydrogenase in gastric cancer. Oncology Letters, 14(5), 5519–5525. https://doi.org/10.3892/ol.2017.6843.
Asting, A. G., Iresjo, B. M., Nilsberth, C., Smedh, U., & Lundholm, K. (2017). Host knockout of E-prostanoid 2 receptors reduces tumor growth and causes major alterations of gene expression in prostaglandin E2-producing tumors. Oncology Letters, 13(1), 476–482. https://doi.org/10.3892/ol.2016.5448.
Du, M., Shi, F., Zhang, H., Xia, S., Zhang, M., Ma, J., et al. (2015). Prostaglandin E2 promotes human cholangiocarcinoma cell proliferation, migration and invasion through the upregulation of beta-catenin expression via EP3-4 receptor. Oncology Reports, 34(2), 715–726. https://doi.org/10.3892/or.2015.4043.
Bazzani, L., Donnini, S., Finetti, F., Christofori, G., & Ziche, M. (2017). PGE2/EP3/SRC signaling induces EGFR nuclear translocation and growth through EGFR ligands release in lung adenocarcinoma cells. Oncotarget, 8(19), 31270–31287. https://doi.org/10.18632/oncotarget.16116.
Yip-Schneider, M. T., Carr, R. A., Wu, H., & Schmidt, C. M. (2017). Prostaglandin E2: a pancreatic fluid biomarker of intraductal papillary mucinous neoplasm dysplasia. Journal of the American College of Surgeons, 225(4), 481–487. https://doi.org/10.1016/j.jamcollsurg.2017.07.521.
Kim, S., Campbell, J., Yoo, W., Taylor, J. A., & Sandler, D. P. (2017). Systemic levels of estrogens and PGE2 synthesis in relation to postmenopausal breast cancer risk. Cancer Epidemiology, Biomarkers & Prevention, 26(3), 383–388. https://doi.org/10.1158/1055-9965.EPI-16-0556.
Reid, G., Wielinga, P., Zelcer, N., van der Heijden, I., Kuil, A., de Haas, M., Wijnholds, J., & Borst, P. (2003). The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proceedings of the National Academy of Sciences of the United States of America, 100(16), 9244–9249. https://doi.org/10.1073/pnas.1033060100.
Kochel, T. J., & Fulton, A. M. (2015). Multiple drug resistance-associated protein 4 (MRP4), prostaglandin transporter (PGT), and 15-hydroxyprostaglandin dehydrogenase (15-PGDH) as determinants of PGE2 levels in cancer. Prostaglandins & Other Lipid Mediators, 116-117, 99–103. https://doi.org/10.1016/j.prostaglandins.2014.11.003.
Kochel, T. J., Reader, J. C., Ma, X., Kundu, N., & Fulton, A. M. (2017). Multiple drug resistance-associated protein (MRP4) exports prostaglandin E2 (PGE2) and contributes to metastasis in basal/triple negative breast cancer. Oncotarget, 8(4), 6540–6554. https://doi.org/10.18632/oncotarget.14145.
Kochel, T. J., Goloubeva, O. G., & Fulton, A. M. (2016). Upregulation of cyclooxygenase-2/prostaglandin E2 (COX-2/PGE2) pathway member multiple drug resistance-associated protein 4 (MRP4) and downregulation of prostaglandin transporter (PGT) and 15-prostaglandin dehydrogenase (15-PGDH) in triple-negative breast cancer. Breast Cancer (Auckl.), 10, 61–70. https://doi.org/10.4137/BCBCR.S38529.
Sugimoto, Y., & Narumiya, S. (2007). Prostaglandin E receptors. The Journal of Biological Chemistry, 282(16), 11613–11617. https://doi.org/10.1074/jbc.R600038200.
Madrigal-Martinez, A., Fernandez-Martinez, A. B., & Lucio Cazana, F. J. (2018). Intracrine prostaglandin E2 pro-tumoral actions in prostate epithelial cells originate from non-canonical pathways. Journal of Cellular Physiology, 233(4), 3590–3602. https://doi.org/10.1002/jcp.26220.
Lian, S., Xia, Y., Ung, T. T., Khoi, P. N., Yoon, H. J., Lee, S. G., Kim, K. K., & Jung, Y. D. (2017). Prostaglandin E2 stimulates urokinase-type plasminogen activator receptor via EP2 receptor-dependent signaling pathways in human AGS gastric cancer cells. Molecular Carcinogenesis, 56(2), 664–680. https://doi.org/10.1002/mc.22524.
Pan, J., Yang, Q., Shao, J., Zhang, L., Ma, J., Wang, Y., Jiang, B. H., Leng, J., & Bai, X. (2016). Cyclooxygenase-2 induced beta1-integrin expression in NSCLC and promoted cell invasion via the EP1/MAPK/E2F-1/FoxC2 signal pathway. Scientific Reports, 6, 33823. https://doi.org/10.1038/srep33823.
Schmidt, A., Sinnett-Smith, J., Young, S., Chang, H. H., Hines, O. J., Dawson, D. W., Rozengurt, E., & Eibl, G. (2017). Direct growth-inhibitory effects of prostaglandin E2 in pancreatic cancer cells in vitro through an EP4/PKA-mediated mechanism. Surgery, 161(6), 1570–1578. https://doi.org/10.1016/j.surg.2016.12.037.
Fernandez-Martinez, A. B., & Lucio-Cazana, J. (2015). Intracellular EP2 prostanoid receptor promotes cancer-related phenotypes in PC3 cells. Cellular and Molecular Life Sciences, 72(17), 3355–3373. https://doi.org/10.1007/s00018-015-1891-5.
Castellone, M. D., Teramoto, H., Williams, B. O., Druey, K. M., & Gutkind, J. S. (2005). Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science, 310(5753), 1504–1510. https://doi.org/10.1126/science.1116221.
Pai, R., Soreghan, B., Szabo, I. L., Pavelka, M., Baatar, D., & Tarnawski, A. S. (2002). Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nature Medicine, 8(3), 289–293. https://doi.org/10.1038/nm0302-289.
Pai, R., Szabo, I. L., Soreghan, B. A., Atay, S., Kawanaka, H., & Tarnawski, A. S. (2001). PGE(2) stimulates VEGF expression in endothelial cells via ERK2/JNK1 signaling pathways. Biochemical and Biophysical Research Communications, 286(5), 923–928. https://doi.org/10.1006/bbrc.2001.5494.
Pang, L. Y., Hurst, E. A., & Argyle, D. J. (2016). Cyclooxygenase-2: a role in cancer stem cell survival and repopulation of cancer cells during therapy. Stem Cells International, 2016, 2048731–2048711. https://doi.org/10.1155/2016/2048731.
Paul, A. G., Chandran, B., & Sharma-Walia, N. (2013). Cyclooxygenase-2-prostaglandin E2-eicosanoid receptor inflammatory axis: a key player in Kaposi’s sarcoma-associated herpes virus associated malignancies. Translational Research, 162(2), 77–92. https://doi.org/10.1016/j.trsl.2013.03.004.
Liao, J., Qian, F., Tchabo, N., Mhawech-Fauceglia, P., Beck, A., Qian, Z., Wang, X., Huss, W. J., Lele, S. B., Morrison, C. D., & Odunsi, K. (2014). Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS One, 9(1), e84941. https://doi.org/10.1371/journal.pone.0084941.
Singh, S. K., Clarke, I. D., Terasaki, M., Bonn, V. E., Hawkins, C., Squire, J., & Dirks, P. B. (2003). Identification of a cancer stem cell in human brain tumors. Cancer Research, 63(18), 5821–5828.
Huang, Q., Li, F., Liu, X., Li, W., Shi, W., Liu, F. F., O'Sullivan, B., He, Z., Peng, Y., Tan, A. C., Zhou, L., Shen, J., Han, G., Wang, X. J., Thorburn, J., Thorburn, A., Jimeno, A., Raben, D., Bedford, J. S., & Li, C. Y. (2011). Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nature Medicine, 17(7), 860–866. https://doi.org/10.1038/nm.2385.
Chimal-Ramirez, G. K., Espinoza-Sanchez, N. A., & Fuentes-Panana, E. M. (2015). A role for the inflammatory mediators cox-2 and metalloproteinases in cancer stemness. Anti-Cancer Agents in Medicinal Chemistry, 15(7), 837–855.
Kundu, N., Ma, X., Kochel, T., Goloubeva, O., Staats, P., Thompson, K., Martin, S., Reader, J., Take, Y., Collin, P., & Fulton, A. (2014). Prostaglandin E receptor EP4 is a therapeutic target in breast cancer cells with stem-like properties. Breast Cancer Research and Treatment, 143(1), 19–31. https://doi.org/10.1007/s10549-013-2779-4.
Boodram, J. N., McGregor, I. J., Bruno, P. M., Cressey, P. B., Hemann, M. T., & Suntharalingam, K. (2016). Breast cancer stem cell potent copper(II)-non-steroidal anti-inflammatory drug complexes. Angewandte Chemie (International Ed. in English), 55(8), 2845–2850. https://doi.org/10.1002/anie.201510443.
Wang, D., Fu, L., Sun, H., Guo, L., & DuBois, R. N. (2015). Prostaglandin E2 promotes colorectal cancer stem cell expansion and metastasis in mice. Gastroenterology, 149(7), 1884–1895 e1884. https://doi.org/10.1053/j.gastro.2015.07.064.
Deng, Y., Su, Q., Mo, J., Fu, X., Zhang, Y., & Lin, E. H. (2013). Celecoxib downregulates CD133 expression through inhibition of the Wnt signaling pathway in colon cancer cells. Cancer Investigation, 31(2), 97–102. https://doi.org/10.3109/07357907.2012.754458.
Guo, Z., Jiang, J. H., Zhang, J., Yang, H. J., Yang, F. Q., Qi, Y. P., Zhong, Y. P., Su, J., Yang, R. R., Li, L. Q., & Xiang, B. D. (2015). COX-2 promotes migration and invasion by the side population of cancer stem cell-like hepatocellular carcinoma cells. Medicine (Baltimore), 94(44), e1806. https://doi.org/10.1097/MD.0000000000001806.
Pang, L. Y., Argyle, S. A., Kamida, A., Morrison, K. O., & Argyle, D. J. (2014). The long-acting COX-2 inhibitor mavacoxib (Trocoxil) has anti-proliferative and pro-apoptotic effects on canine cancer cell lines and cancer stem cells in vitro. BMC Veterinary Research, 10, 184. https://doi.org/10.1186/s12917-014-0184-9.
Tafani, M., Di Vito, M., Frati, A., Pellegrini, L., De Santis, E., Sette, G., et al. (2011). Pro-inflammatory gene expression in solid glioblastoma microenvironment and in hypoxic stem cells from human glioblastoma. Journal of Neuroinflammation, 8, 32. https://doi.org/10.1186/1742-2094-8-32.
Kurtova, A. V., Xiao, J., Mo, Q., Pazhanisamy, S., Krasnow, R., Lerner, S. P., Chen, F., Roh, T. T., Lay, E., Ho, P. L., & Chan, K. S. (2015). Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature, 517(7533), 209–213. https://doi.org/10.1038/nature14034.
Hou, P. C., Li, Y. H., Lin, S. C., Lee, J. C., Lin, B. W., Liou, J. P., et al. (2017). Hypoxia-induced downregulation of DUSP-2 phosphatase drives colon cancer stemness. Cancer Research, 77(16), 4305–4316. https://doi.org/10.1158/0008-5472.CAN-16-2990.
Fang, D., & Kitamura, H. (2018). Cancer stem cells and epithelial-mesenchymal transition in urothelial carcinoma: possible pathways and potential therapeutic approaches. International Journal of Urology, 25(1), 7–17. https://doi.org/10.1111/iju.13404.
Wu, M., Guan, J., Li, C., Gunter, S., Nusrat, L., Ng, S., et al. (2017). Aberrantly activated Cox-2 and Wnt signaling interact to maintain cancer stem cells in glioblastoma. Oncotarget, 8(47), 82217–82230. https://doi.org/10.18632/oncotarget.19283.
Liu, X., Ji, Q., Ye, N., Sui, H., Zhou, L., Zhu, H., Fan, Z., Cai, J., & Li, Q. (2015). Berberine inhibits invasion and metastasis of colorectal cancer cells via COX-2/PGE2 mediated JAK2/STAT3 signaling pathway. PLoS One, 10(5), e0123478. https://doi.org/10.1371/journal.pone.0123478.
Hay, E. D. (1995). An overview of epithelio-mesenchymal transformation. Acta Anatomica (Basel), 154(1), 8–20.
Kalluri, R., & Weinberg, R. A. (2009). The basics of epithelial-mesenchymal transition. The Journal of Clinical Investigation, 119(6), 1420–1428. https://doi.org/10.1172/JCI39104.
Thiery, J. P. (2003). Epithelial-mesenchymal transitions in development and pathologies. Current Opinion in Cell Biology, 15(6), 740–746.
Mathenge, E. G., Dean, C. A., Clements, D., Vaghar-Kashani, A., Photopoulos, S., Coyle, K. M., Giacomantonio, M., Malueth, B., Nunokawa, A., Jordan, J., Lewis, J. D., Gujar, S. A., Marcato, P., Lee, P. W. K., & Giacomantonio, C. A. (2014). Core needle biopsy of breast cancer tumors increases distant metastases in a mouse model. Neoplasia, 16(11), 950–960. https://doi.org/10.1016/j.neo.2014.09.004.
Aktipis, C. A., Kwan, V. S., Johnson, K. A., Neuberg, S. L., & Maley, C. C. (2011). Overlooking evolution: a systematic analysis of cancer relapse and therapeutic resistance research. PLoS One, 6(11), e26100. https://doi.org/10.1371/journal.pone.0026100.
Du, B., & Shim, J. S. (2016). Targeting epithelial-mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules, 21(7). https://doi.org/10.3390/molecules21070965.
Gonzalez, D. M., & Medici, D. (2014). Signaling mechanisms of the epithelial-mesenchymal transition. Science Signaling, 7(344), re8. https://doi.org/10.1126/scisignal.2005189.
St John, M. A. (2015). Inflammatory mediators drive metastasis and drug resistance in head and neck squamous cell carcinoma. Laryngoscope, 125(Suppl 3), S1–S11. https://doi.org/10.1002/lary.24998.
Bocca, C., Ievolella, M., Autelli, R., Motta, M., Mosso, L., Torchio, B., Bozzo, F., Cannito, S., Paternostro, C., Colombatto, S., Parola, M., & Miglietta, A. (2014). Expression of Cox-2 in human breast cancer cells as a critical determinant of epithelial-to-mesenchymal transition and invasiveness. Expert Opinion on Therapeutic Targets, 18(2), 121–135. https://doi.org/10.1517/14728222.2014.860447.
Fujii, R., Imanishi, Y., Shibata, K., Sakai, N., Sakamoto, K., Shigetomi, S., Habu, N., Otsuka, K., Sato, Y., Watanabe, Y., Ozawa, H., Tomita, T., Kameyama, K., Fujii, M., & Ogawa, K. (2014). Restoration of E-cadherin expression by selective Cox-2 inhibition and the clinical relevance of the epithelial-to-mesenchymal transition in head and neck squamous cell carcinoma. Journal of Experimental & Clinical Cancer Research, 33, 40. https://doi.org/10.1186/1756-9966-33-40.
Liu, X. J., Chen, Z. F., Li, H. L., Hu, Z. N., Liu, M., Tian, A. P., Zhao, D., Wu, J., Zhou, Y. N., & Qiao, L. (2013). Interaction between cyclooxygenase-2, Snail, and E-cadherin in gastric cancer cells. World Journal of Gastroenterology, 19(37), 6265–6271. https://doi.org/10.3748/wjg.v19.i37.6265.
Bozzo, F., Bassignana, A., Lazzarato, L., Boschi, D., Gasco, A., Bocca, C., & Miglietta, A. (2009). Novel nitro-oxy derivatives of celecoxib for the regulation of colon cancer cell growth. Chemico-Biological Interactions, 182(2–3), 183–190. https://doi.org/10.1016/j.cbi.2009.08.006.
Dinicola, S., Masiello, M. G., Proietti, S., Coluccia, P., Fabrizi, G., Catizone, A., Ricci, G., de Toma, G., Bizzarri, M., & Cucina, A. (2018). Nicotine increases colon cancer cell migration and invasion through epithelial to mesenchymal transition (EMT): COX-2 involvement. Journal of Cellular Physiology, 233(6), 4935–4948. https://doi.org/10.1002/jcp.26323.
Jansen, S. R., Holman, R., Hedemann, I., Frankes, E., Elzinga, C. R., Timens, W., et al. (2015). Prostaglandin E2 promotes MYCN non-amplified neuroblastoma cell survival via beta-catenin stabilization. Journal of Cellular and Molecular Medicine, 19(1), 210–226. https://doi.org/10.1111/jcmm.12418.
Che, D., Zhang, S., Jing, Z., Shang, L., Jin, S., Liu, F., Shen, J., Li, Y., Hu, J., Meng, Q., & Yu, Y. (2017). Macrophages induce EMT to promote invasion of lung cancer cells through the IL-6-mediated COX-2/PGE2/beta-catenin signalling pathway. Molecular Immunology, 90, 197–210. https://doi.org/10.1016/j.molimm.2017.06.018.
Jansen, S. R., Poppinga, W. J., de Jager, W., Lezoualc'h, F., Cheng, X., Wieland, T., et al. (2016). Epac1 links prostaglandin E2 to beta-catenin-dependent transcription during epithelial-to-mesenchymal transition. Oncotarget, 7(29), 46354–46370. https://doi.org/10.18632/oncotarget.10128.
Majumder, M., Dunn, L., Liu, L., Hasan, A., Vincent, K., Brackstone, M., Hess, D., & Lala, P. K. (2018). COX-2 induces oncogenic micro RNA miR655 in human breast cancer. Scientific Reports, 8(1), 327. https://doi.org/10.1038/s41598-017-18612-3.
Li, P., Shan, J. X., Chen, X. H., Zhang, D., Su, L. P., Huang, X. Y., Yu, B. Q., Zhi, Q. M., Li, C. L., Wang, Y. Q., Tomei, S., Cai, Q., Ji, J., Li, J. F., Chouchane, L., Yu, Y. Y., Sun, F. Z., Xu, Z. H., Liu, B. Y., & Zhu, Z. G. (2015). Epigenetic silencing of microRNA-149 in cancer-associated fibroblasts mediates prostaglandin E2/interleukin-6 signaling in the tumor microenvironment. Cell Research, 25(5), 588–603. https://doi.org/10.1038/cr.2015.51.
Deorukhkar, A., & Krishnan, S. (2010). Targeting inflammatory pathways for tumor radiosensitization. Biochemical Pharmacology, 80(12), 1904–1914. https://doi.org/10.1016/j.bcp.2010.06.039.
Jimenez-Garduno, A. M., Mendoza-Rodriguez, M. G., Urrutia-Cabrera, D., Dominguez-Robles, M. C., Perez-Yepez, E. A., Ayala-Sumuano, J. T., et al. (2017). IL-1beta induced methylation of the estrogen receptor ERalpha gene correlates with EMT and chemoresistance in breast cancer cells. Biochemical and Biophysical Research Communications, 490(3), 780–785. https://doi.org/10.1016/j.bbrc.2017.06.117.
Jung, K., Heishi, T., Khan, O. F., Kowalski, P. S., Incio, J., Rahbari, N. N., Chung, E., Clark, J. W., Willett, C. G., Luster, A. D., Yun, S. H., Langer, R., Anderson, D. G., Padera, T. P., Jain, R. K., & Fukumura, D. (2017). Ly6Clo monocytes drive immunosuppression and confer resistance to anti-VEGFR2 cancer therapy. The Journal of Clinical Investigation, 127(8), 3039–3051. https://doi.org/10.1172/JCI93182.
Gonda, K., Shibata, M., Ohtake, T., Matsumoto, Y., Tachibana, K., Abe, N., Ohto, H., Sakurai, K., & Takenoshita, S. (2017). Myeloid-derived suppressor cells are increased and correlated with type 2 immune responses, malnutrition, inflammation, and poor prognosis in patients with breast cancer. Oncology Letters, 14(2), 1766–1774. https://doi.org/10.3892/ol.2017.6305.
Liu, B., Qu, L., & Yan, S. (2015). Cyclooxygenase-2 promotes tumor growth and suppresses tumor immunity. Cancer Cell International, 15, 106. https://doi.org/10.1186/s12935-015-0260-7.
Zelenay, S., van der Veen, A. G., Bottcher, J. P., Snelgrove, K. J., Rogers, N., Acton, S. E., et al. (2015). Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell, 162(6), 1257–1270. https://doi.org/10.1016/j.cell.2015.08.015.
Bottcher, J. P., Bonavita, E., Chakravarty, P., Blees, H., Cabeza-Cabrerizo, M., Sammicheli, S., et al. (2018). NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell, 172(5), 1022–1037 e1014. https://doi.org/10.1016/j.cell.2018.01.004.
Roberts, E. W., Broz, M. L., Binnewies, M., Headley, M. B., Nelson, A. E., Wolf, D. M., Kaisho, T., Bogunovic, D., Bhardwaj, N., & Krummel, M. F. (2016). Critical role for CD103(+)/CD141(+) dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell, 30(2), 324–336. https://doi.org/10.1016/j.ccell.2016.06.003.
Salmon, H., Idoyaga, J., Rahman, A., Leboeuf, M., Remark, R., Jordan, S., Casanova-Acebes, M., Khudoynazarova, M., Agudo, J., Tung, N., Chakarov, S., Rivera, C., Hogstad, B., Bosenberg, M., Hashimoto, D., Gnjatic, S., Bhardwaj, N., Palucka, A. K., Brown, B. D., Brody, J., Ginhoux, F., & Merad, M. (2016). Expansion and activation of CD103(+) dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity, 44(4), 924–938. https://doi.org/10.1016/j.immuni.2016.03.012.
Spranger, S., Dai, D., Horton, B., & Gajewski, T. F. (2017). Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell, 31(5), 711–723 e714. https://doi.org/10.1016/j.ccell.2017.04.003.
Broz, M. L., Binnewies, M., Boldajipour, B., Nelson, A. E., Pollack, J. L., Erle, D. J., Barczak, A., Rosenblum, M. D., Daud, A., Barber, D. L., Amigorena, S., van’t Veer, L. J., Sperling, A. I., Wolf, D. M., & Krummel, M. F. (2014). Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell, 26(6), 938. https://doi.org/10.1016/j.ccell.2014.11.010.
Ruffell, B., Chang-Strachan, D., Chan, V., Rosenbusch, A., Ho, C. M., Pryer, N., et al. (2014). Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell, 26(5), 623–637. https://doi.org/10.1016/j.ccell.2014.09.006.
Sinha, P., Clements, V. K., Fulton, A. M., & Ostrand-Rosenberg, S. (2007). Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Research, 67(9), 4507–4513. https://doi.org/10.1158/0008-5472.CAN-06-4174.
Wang, M. T., Honn, K. V., & Nie, D. (2007). Cyclooxygenases, prostanoids, and tumor progression. Cancer Metastasis Reviews, 26(3–4), 525–534. https://doi.org/10.1007/s10555-007-9096-5.
Sreeramkumar, V., Fresno, M., & Cuesta, N. (2012). Prostaglandin E2 and T cells: friends or foes? Immunology and Cell Biology, 90(6), 579–586. https://doi.org/10.1038/icb.2011.75.
Galvan, G. C., Johnson, C. B., Price, R. S., Liss, M. A., Jolly, C. A., & deGraffenried, L. A. (2017). Effects of obesity on the regulation of macrophage population in the prostate tumor microenvironment. Nutrition and Cancer, 69(7), 996–1002. https://doi.org/10.1080/01635581.2017.1359320.
Prima, V., Kaliberova, L. N., Kaliberov, S., Curiel, D. T., & Kusmartsev, S. (2017). COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proceedings of the National Academy of Sciences of the United States of America, 114(5), 1117–1122. https://doi.org/10.1073/pnas.1612920114.
Olesch, C., Sha, W., Angioni, C., Sha, L. K., Acaf, E., Patrignani, P., et al. (2015). MPGES-1-derived PGE2 suppresses CD80 expression on tumor-associated phagocytes to inhibit anti-tumor immune responses in breast cancer. Oncotarget, 6(12), 10284–10296. https://doi.org/10.18632/oncotarget.3581.
Furuya, H., Tamashiro, P. M., Shimizu, Y., Iino, K., Peres, R., Chen, R., Sun, Y., Hannun, Y. A., Obeid, L. M., & Kawamori, T. (2017). Sphingosine kinase 1 expression in peritoneal macrophages is required for colon carcinogenesis. Carcinogenesis, 38(12), 1218–1227. https://doi.org/10.1093/carcin/bgx104.
Snijdewint, F. G., Kalinski, P., Wierenga, E. A., Bos, J. D., & Kapsenberg, M. L. (1993). Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. Journal of Immunology, 150(12), 5321–5329.
Huang, M., Stolina, M., Sharma, S., Mao, J. T., Zhu, L., Miller, P. W., Wollman, J., Herschman, H., & Dubinett, S. M. (1998). Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: up-regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Research, 58(6), 1208–1216.
Wu, A. A., Drake, V., Huang, H. S., Chiu, S., & Zheng, L. (2015). Reprogramming the tumor microenvironment: tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology, 4(7), e1016700. https://doi.org/10.1080/2162402X.2015.1016700.
Stolina, M., Sharma, S., Lin, Y., Dohadwala, M., Gardner, B., Luo, J., Zhu, L., Kronenberg, M., Miller, P. W., Portanova, J., Lee, J. C., & Dubinett, S. M. (2000). Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. Journal of Immunology, 164(1), 361–370.
Miao, J., Lu, X., Hu, Y., Piao, C., Wu, X., Liu, X., et al. (2017). Prostaglandin E2 and PD-1 mediated inhibition of antitumor CTL responses in the human tumor microenvironment. Oncotarget, 8(52), 89802–89810. https://doi.org/10.18632/oncotarget.21155.
Krishnan, A. V., Moreno, J., Nonn, L., Swami, S., Peehl, D. M., & Feldman, D. (2007). Calcitriol as a chemopreventive and therapeutic agent in prostate cancer: role of anti-inflammatory activity. Journal of Bone and Mineral Research, 22(Suppl 2), V74–V80. https://doi.org/10.1359/jbmr.07s213.
Hsu, H. H., Chen, M. C., Day, C. H., Lin, Y. M., Li, S. Y., Tu, C. C., Padma, V. V., Shih, H. N., Kuo, W. W., & Huang, C. Y. (2017). Thymoquinone suppresses migration of LoVo human colon cancer cells by reducing prostaglandin E2 induced COX-2 activation. World Journal of Gastroenterology, 23(7), 1171–1179. https://doi.org/10.3748/wjg.v23.i7.1171.
Ghorab, M. M., El-Gaby, M. S. A., Alsaid, M. S., Elshaier, Y., Soliman, A. M., El-Senduny, F. F., et al. (2017). Novel thiourea derivatives bearing sulfonamide moiety as anticancer agents through COX-2 inhibition. Anti-Cancer Agents in Medicinal Chemistry, 17(10), 1411–1425. https://doi.org/10.2174/1871520617666170327153735.
Qin, G., Xu, F., Qin, T., Zheng, Q., Shi, D., Xia, W., et al. (2015). Palbociclib inhibits epithelial-mesenchymal transition and metastasis in breast cancer via c-Jun/COX-2 signaling pathway. Oncotarget, 6(39), 41794–41808. https://doi.org/10.18632/oncotarget.5993.
Pun, I. H., Chan, D., Chan, S. H., Chung, P. Y., Zhou, Y. Y., Law, S., et al. (2017). Anti-cancer effects of a novel quinoline derivative 83b1 on human esophageal squamous cell carcinoma through down-regulation of COX-2 mRNA and PGE2. Cancer Research and Treatment, 49(1), 219–229. https://doi.org/10.4143/crt.2016.190.
Verma, A., Ahmed, B., Anwar, F., Rahman, M., Patel, D. K., Kaithwas, G., Rani, R., Bhatt, P. C., & Kumar, V. (2017). Novel glycoside from Wedelia calendulacea inhibits diethyl nitrosamine-induced renal cancer via downregulating the COX-2 and PEG2 through nuclear factor-kappaB pathway. Inflammopharmacology, 25(1), 159–175. https://doi.org/10.1007/s10787-017-0310-y.
Lin, Y. M., Kuo, W. W., Velmurugan, B. K., Hsien, H. H., Hsieh, Y. L., Hsu, H. H., Tu, C. C., Bau, D. T., Viswanadha, V. P., & Huang, C. Y. (2016). Helioxanthin suppresses the cross talk of COX-2/PGE2 and EGFR/ERK pathway to inhibit arecoline-induced oral cancer cell (T28) proliferation and blocks tumor growth in xenografted nude mice. Environmental Toxicology, 31(12), 2045–2056. https://doi.org/10.1002/tox.22204.
Seira, N., Yanagisawa, N., Suganami, A., Honda, T., Wasai, M., Regan, J. W., Fukushima, K., Yamaguchi, N., Tamura, Y., Arai, T., Murayama, T., & Fujino, H. (2017). Anti-cancer effects of MW-03, a novel indole compound, by inducing 15-hydroxyprostaglandin dehydrogenase and cellular growth inhibition in the LS174T human colon cancer cell line. Biological & Pharmaceutical Bulletin, 40(10), 1806–1812. https://doi.org/10.1248/bpb.b17-00458.
Choi, S. H., Kim, B. G., Robinson, J., Fink, S., Yan, M., Sporn, M. B., Markowitz, S. D., & Letterio, J. J. (2014). Synthetic triterpenoid induces 15-PGDH expression and suppresses inflammation-driven colon carcinogenesis. The Journal of Clinical Investigation, 124(6), 2472–2482. https://doi.org/10.1172/JCI69672.
Kangwan, N., Kim, Y. J., Han, Y. M., Jeong, M., Park, J. M., Go, E. J., et al. (2016). Sonic Hedgehog inhibitors prevent colitis-associated cancer via orchestrated mechanisms of IL-6/gp130 inhibition, 15-PGDH induction, Bcl-2 abrogation, and tumorsphere inhibition. Oncotarget, 7(7), 7667–7682. https://doi.org/10.18632/oncotarget.6765.
Tai, H. H., Chi, X., & Tong, M. (2011). Regulation of 15-hydroxyprostaglandin dehydrogenase (15-PGDH) by non-steroidal anti-inflammatory drugs (NSAIDs). Prostaglandins & Other Lipid Mediators, 96(1–4), 37–40. https://doi.org/10.1016/j.prostaglandins.2011.06.005.
Chi, X., Freeman, B. M., Tong, M., Zhao, Y., & Tai, H. H. (2009). 15-Hydroxyprostaglandin dehydrogenase (15-PGDH) is up-regulated by flurbiprofen and other non-steroidal anti-inflammatory drugs in human colon cancer HT29 cells. Archives of Biochemistry and Biophysics, 487(2), 139–145. https://doi.org/10.1016/j.abb.2009.05.017.
Chi, X., & Tai, H. H. (2010). Interleukin-4 up-regulates 15-hydroxyprostaglandin dehydrogenase (15-PGDH) in human lung cancer cells. Experimental Cell Research, 316(14), 2251–2259.
Xun, C. Q., Tian, Z. G., & Tai, H. H. (1991). Stimulation of synthesis de novo of NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase in human promyelocytic leukaemia (HL-60) cells by phorbol ester. The Biochemical Journal, 279(Pt 2), 553–558.
Kitamura, T., Itoh, M., Noda, T., Tani, K., Kobayashi, M., Maruyama, T., Kobayashi, K., Ohuchida, S., Sugimura, T., & Wakabayashi, K. (2003). Combined effects of prostaglandin E receptor subtype EP1 and subtype EP4 antagonists on intestinal tumorigenesis in adenomatous polyposis coli gene knockout mice. Cancer Science, 94(7), 618–621.
Kitamura, T., Itoh, M., Noda, T., Matsuura, M., & Wakabayashi, K. (2004). Combined effects of cyclooxygenase-1 and cyclooxygenase-2 selective inhibitors on intestinal tumorigenesis in adenomatous polyposis coli gene knockout mice. International Journal of Cancer, 109(4), 576–580. https://doi.org/10.1002/ijc.20012.
Niho, N., Mutoh, M., Kitamura, T., Takahashi, M., Sato, H., Yamamoto, H., Maruyama, T., Ohuchida, S., Sugimura, T., & Wakabayashi, K. (2005). Suppression of azoxymethane-induced colon cancer development in rats by a prostaglandin E receptor EP1-selective antagonist. Cancer Science, 96(5), 260–264. https://doi.org/10.1111/j.1349-7006.2005.00047.x.
Jin, J., Chang, Y., Wei, W., He, Y. F., Hu, S. S., Wang, D., & Wu, Y. J. (2012). Prostanoid EP1 receptor as the target of (−)-epigallocatechin-3-gallate in suppressing hepatocellular carcinoma cells in vitro. Acta Pharmacologica Sinica, 33(5), 701–709. https://doi.org/10.1038/aps.2012.13.
Cheuk, I. W., Shin, V. Y., Siu, M. T., Tsang, J. Y., Ho, J. C., Chen, J., Tse, G. M., Wang, X., & Kwong, A. (2015). Association of EP2 receptor and SLC19A3 in regulating breast cancer metastasis. American Journal of Cancer Research, 5(11), 3389–3399.
Ikawa, Y., Fujino, H., Otake, S., & Murayama, T. (2012). Indomethacin antagonizes EP(2) prostanoid receptor activation in LS174T human colon cancer cells. European Journal of Pharmacology, 680(1–3), 16–21. https://doi.org/10.1016/j.ejphar.2012.01.033.
Holt, D. M., Ma, X., Kundu, N., Collin, P. D., & Fulton, A. M. (2012). Modulation of host natural killer cell functions in breast cancer via prostaglandin E2 receptors EP2 and EP4. Journal of Immunotherapy, 35(2), 179–188. https://doi.org/10.1097/CJI.0b013e318247a5e9.
Li, C., Liu, X., Liu, Y., Zhang, E., Medepalli, K., Masuda, K., Li, N., Wikenheiser-Brokamp, K. A., Osterburg, A., Borchers, M. T., Kopras, E. J., Plas, D. R., Sun, J., Franz, D. N., Capal, J. K., Mays, M., Sun, Y., Kwiatkowski, D. J., Alayev, A., Holz, M. K., Krueger, D. A., Siroky, B. J., & Yu, J. J. (2017). Tuberin regulates prostaglandin receptor-mediated viability, via Rheb, in mTORC1-hyperactive cells. Molecular Cancer Research, 15(10), 1318–1330. https://doi.org/10.1158/1541-7786.MCR-17-0077.
Fang, T., Hou, J., He, M., Wang, L., Zheng, M., Wang, X., & Xia, J. (2016). Actinidia chinensis planch root extract (acRoots) inhibits hepatocellular carcinoma progression by inhibiting EP3 expression. Cell Biology and Toxicology, 32(6), 499–511. https://doi.org/10.1007/s10565-016-9351-z.
Zhu, J., Trillsch, F., Mayr, D., Kuhn, C., Rahmeh, M., Hofmann, S., et al. (2018). Prostaglandin receptor EP3 regulates cell proliferation and migration with impact on survival of endometrial cancer patients. Oncotarget, 9(1), 982–994. https://doi.org/10.18632/oncotarget.23140.
Hoshikawa, H., Goto, R., Mori, T., Mitani, T., & Mori, N. (2009). Expression of prostaglandin E2 receptors in oral squamous cell carcinomas and growth inhibitory effects of an EP3 selective antagonist, ONO-AE3-240. International Journal of Oncology, 34(3), 847–852.
Ma, X., Holt, D., Kundu, N., Reader, J., Goloubeva, O., Take, Y., & Fulton, A. M. (2013). A prostaglandin E (PGE) receptor EP4 antagonist protects natural killer cells from PGE2-mediated immunosuppression and inhibits breast cancer metastasis. Oncoimmunology, 2(1), e22647. https://doi.org/10.4161/onci.22647.
Robertson, F. M., Simeone, A. M., Mazumdar, A., Shah, A. H., McMurray, J. S., Ghosh, S., et al. (2008). Molecular and pharmacological blockade of the EP4 receptor selectively inhibits both proliferation and invasion of human inflammatory breast cancer cells. Journal of Experimental Therapeutics & Oncology, 7(4), 299–312.
Majumder, M., Xin, X., Liu, L., Girish, G. V., & Lala, P. K. (2014). Prostaglandin E2 receptor EP4 as the common target on cancer cells and macrophages to abolish angiogenesis, lymphangiogenesis, metastasis, and stem-like cell functions. Cancer Science, 105(9), 1142–1151. https://doi.org/10.1111/cas.12475.
Parida, S., Parekh, A., Dey, G., Ghosh, S. C., & Mandal, M. (2015). Molecular inhibition of prostaglandin E2 with GW627368X: therapeutic potential and preclinical safety assessment in mouse sarcoma model. Cancer Biology & Therapy, 16(6), 922–932. https://doi.org/10.1080/15384047.2015.1040953.
Acknowledgements
This work was supported for Jun Jiang by the National Natural Science Foundation of China (NSFC) (Grant Nos.: 81772704 and 81172442) and Army Healthcare Foundation of China (Grant No.: 17BJZ13) and Dali Tong by NSFC (Grant No.: 81402120).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Dali Tong and Qiuli Liu contribute equally to this review.
Rights and permissions
About this article
Cite this article
Tong, D., Liu, Q., Wang, La. et al. The roles of the COX2/PGE2/EP axis in therapeutic resistance. Cancer Metastasis Rev 37, 355–368 (2018). https://doi.org/10.1007/s10555-018-9752-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-018-9752-y