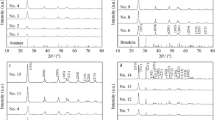
A novel multi-gelation method to prepare TiO2 nano-particle photocatalysts showed good performance in controlling the important parameters determining the photocatalytic reactivity, i.e., the particle size, surface area, crystallinity, pore-volume, pore-diameter as well as the anatase and rutile phase composition of the catalysts. In particular, this method at higher pH swing times could prevent the phase transition from anatase to rutile, leading to higher photocatalytic activity. By adopting variations in the pH swing, the TiO2 nano-particle photocatalysts showed significantly higher photocatalytic reactivity for the complete oxidation of 2-propanol diluted with water into CO2 and H2O. It can be considered a viable alternative method for the preparation of high performance TiO2 nano-particle photocatalysts for widespread commercial applications.
Similar content being viewed by others
References
M. Anpo (2004) Bull. Chem. Soc. Jpn. 77 1427
M. Anpo M. Takeuchi (2003) J. Catal. 216 505
M. Anpo H. Yamashita (2004) Catal. Surv. Asia 8 35
J.L. Zhang Y. Hu M. Matsuoka H. Yamashita M. Minagawa H. Hidaka M. Anpo (2001) J. Phys. Chem. B 105 8395
M. Anpo (1989) Res. Chem. Intermed. 11 67
M. Anpo T. Shima S. Kodama Y. Kubokawa (1987) J. Phys. Chem. 91 4305
M. Yan F. Chen J. Zhang M. Anpo (2005) J. Phys. Chem. B 109 8673
J.M. Herrmann C. Guillard J. Disdier C. Lehaut S. Malato J. Blanco (2002) Appl. Catal. B 35 281
C.Y. Wang J. Rabani D.W. Bahnemann J.K. Dohrmann (2002) J. Photochem. Photobiol. A 148 169
S. Bakardjieva J. Subrt V. Stengl M.J. Dianez M.J. Sayagues (2005) Appl. Catal. B 58 193
B. Neppolian H.S. Jie J.P. Ahn J.K. Park M. Anpo (2004) Chem. Lett. 33 1562
M.A. Fox M.T. Gulay (1993) Chem. Rev. 93 341
H. Yamashita Y. Ichihashi M. Harada G. Stewart M.A. Fox M. Anpo (1996) J. Catal. 158 97
J.G. Yu J.C. Yu B. Cheng S.K. Hark K. Iu (2003) J. Solid State Chem. 174 372
K.N.P. Kumar K. Keizer A.J. Burggraaf (1993) J. Mater. Chem. 3 917
J. Yang Y.X. Huang J.M.F. Ferreria (1997) J. Mater. Sci. Lett. 16 1933
J. Kim K.C. Song S. Foncillas S.E. Pratsinis (2001) J. Eur. Ceram. Soc. 21 2863
M. Anpo T. Kawamura S. Kodama K. Maruya T. Onishi (1988) J. Phys. Chem. 92 438
M. Anpo H. Nakaya S. Kodama Y. Kubokawa K. Domen T. Onishi (1986) J. Phys. Chem. 90 1633
H. Li J. Zhu G. Li Y. Wan (2004) Chem. Lett. 33 574
D.C. Hurum A.G. Agrios K.A. Gray T. Rajh M.C. Thurnauer (2003) J. Phys. Chem. B 107 4545
U. Stafford K.A. Gray P.V. Kamat V. Varma (1993) Chem. Phys. Lett. 205 55
T. Ohno K. Tokieda S. Higashida M. Matsumura (2003) Appl. Catal. A 244 383
T. Ohno K. Sarukawa M. Matsumura (2001) J. Phys. Chem. B 105 2417
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Neppolian, B., Yamashita, H., Okada, Y. et al. Preparation of Unique TiO2 Nano-particle Photocatalysts by a Multi-gelation Method for Control of the Physicochemical Parameters and Reactivity. Catal Lett 105, 111–117 (2005). https://doi.org/10.1007/s10562-005-8013-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10562-005-8013-1