Abstract
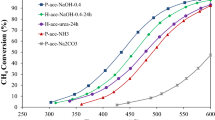
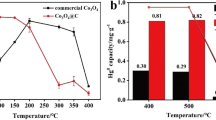
Without use of any surfactant or oxidant, a series of Co3O4 catalysts have been prepared from cobalt nitrate aqueous solution via a very simple liquid-precipitation method with ammonium acid carbonate followed by calcination at various temperatures. The catalytic performance of the Co3O4 for CO oxidation has been studied with a continuous flowing laboratory microreactor system. The results show that the CO conversion of all the samples can reach 100% at ambient temperature. The catalyst calcined at 300 °C is able to maintain its activity for CO complete oxidation more than 500 min at 25 °C and about 240 min even at −78 °C. High reaction temperature results in a high catalytic stability, while the catalytic stability decreases with further increasing the reaction temperature. Characterizations with X-ray powder diffraction and transmission electron microscopy suggest that all the samples exist as a pure Co3O4 phase with the spinel structure, the samples are apt to aggregate and the specific surface area gradually decreases with increasing the calcination temperature, which directly leads to the decrease of catalytic stability. Furthermore, the amount of active oxygen species measured by CO titration experiments appears to be critical for catalytic performance.






Similar content being viewed by others
References
Gardner S.D., Hoflund G.B., Upchurch B.T., Schryer D.R., Kielin E.J., Schryer J. (1991) J. Catal. 129:114
Lamb A.B., Bray W.C., Frazer J.C.W. (1920) Ind. Eng. Chem. 12:213
Yamaura H., Moriya K., Miura N., Yamazoe N. (2000) Sens. Actuators B 65:39
Funazaki N., Asano Y., Yamashita S., Kobayashi T., Haruta M. (1993) Sens. Actuators B 13–14:536
Tripathi A.K., Gupta N.M., Chatterji U.K., Iyer R.M. (1992) Indian J. Technol. 30:107
Thormählen P., Fridell E., Cruise N., Skoglundh M., Palmqvist A. (2001) Appl. Catal. B 31:1
Shelef M., McCabe R.W. (2000) Catal. Today 62:35
Kim D.H., Lim M.S. (2002) Appl. Catal. A 224:27
Snytniko P.V., Sobyanin V.A., Belyaev V.D., Tsyrulniko P.G., Shitova N.B., Shlyapin D.A. (2003) Appl. Catal. A 239:149
Dong G.L., Wang J.G., Gao Y.B., Chen S.Y. (1999) Catal. Lett. 58:37
Bi Y.U., Lu G.X. (2003) Appl. Catal. B 41:279
Bera P., Gayen A., Hegde M.S., Lalla N.P., Spadaro L., Frusteri F., Arena F. (2003) J. Phys. Chem. B 107:6122
Margitfalvi J.L., Borbáth I., Hegedűs M., Tfirst E., Gőbölös S., Lázár K. (2000) J. Catal. 196:200
Daniel M.C., Astruc D. Chem. Rev. 104 (2004) 341, and references cited therein
Jia M.L., Shen Y.N., Li C.Y., Bao Z.R.G.T., Sheng S.S. (2005) Catal. Lett. 99:235
Centeno M.Á., Portales C., Carrizosa I., Odriozola J.A. (2005) Catal. Lett. 102:289
Jain A., Zhao X., Kjergaard S., Stagg-Williams S.M. (2005) Catal. Lett. 104:191
Moreau F., Bond G.C. (2006) Catal. Today 114:362
Moreau F., Bond G.C., Taylor A.O. (2004) Chem. Comm. 1642
Chiang C.W., Wang A.Q., Wan B.Z., Mou C.Y. (2005) J. Phys. Chem. B 109:18042
Luo M.F., Zhong Y.J., Yuan X.X., Zheng X.M. (1997) Appl. Catal. A 162:121
Hutchings G.J., Mirzaei A.A., Joyner R.W., Siddiqui M.R.H., Taylor S.H. (1998) Appl. Catal. A 166:143
S.H. Taylor, G.J. Hutchings, A.A. Mirzaei (1999) Chem. Comm. 1373
Whittle D.M., Mirzaei A.A., Hargreaves J.S.J., Joyner R.W., Kiely C.J., Taylor S.H., Hutchings G.J. (2002) Phys. Chem. Chem. Phys. 4:5915
Bae C.M., Ko J.B., Kim D.H. (2005) Catal. Comm. 6:507
Yu Y., Yung F. (1974) J. Catal. 33:108
Jia M.J., Zhang W.X., Tao Y.G., Wang G.Y., Cui X.H., Zhang C.L., Wu T.H. (1999) Chem. J. Chin. Univ. 20:637 (in Chinese)
Lin H.K., Chiu H.C., Tsai H.C., Chien S.H., Wang C.B. (2003) Catal. Lett. 88:169
Lin H.K., Wang C.B., Chiu H.C., Chien S.H. (2003) Catal. Lett. 86:63
Wang C.B., Tang C.W., Gau S.J., Chien S.H. (2005) Catal. Lett. 101:59
Cunningham D.A.H., Kobayashi T., Kamijo N., Haruta M. (1994) Catal. Lett. 25:257
Jansson J. (2000) J. Catal. 194:55
Jansson J., Anders E.C.P., Fridell E., Skoglundh M., Österlund L., Thormählen P., Langer V. (2002) J. Catal. 211:387
Thormählen P., Skoglundh M., Fridell E., Andersson B.(1999) J. Catal. 188:300
Zheng X.C., Wu S.H., Wang S.P., Wang S.R., Zhang S.M., Huang W.P. (2005) Appl. Catal. A 283:217
Chen Y.Z., Liaw B.J., Huang C.W. (2006) Appl. Catal. A 302:168
Krämer M., Schmidt T., Stöwe K., Maier W.F. (2006) Appl. Catal. A 302:257
Drago R.S., Jurczyk K., Singh D.J., Young V. (1995) Appl. Catal. B 6:155
Steen E.V., Schulz H. (1999) Appl. Catal. A 186:309
Zhang Z.L., Geng H.R., Zheng L.S., Du B. (2005) J. Alloys. Compd. 392:317
Schmidt-Szałowski K., Krawczyk K., Petryk J. (1998) Appl. Catal. A 175:147
Langford J.I., Wilson A.J.C. (1978) J. Appl. Crystallogr. 11:102
Gaddsden J.A. (1975) Infrared spectra of minerals and related inorganic compounds. Butterworth, London, p. 44
Spenser C., Schroeder D. (1974) Phys. Rev. B 9:3658
Andrushkevich T., Boreskov G., Popovskii V., Pliasova L., Karakchiev L., Ostankovitch A. (1968) Kinet. Katal. 6:1244
Christoskova St.G., Stoyanova M., Georgieva M., Mehandjiev D. (1999) Mater. Chem. Phys. 60:39
Singh R.N., Pandey J.P., Singh N.K., Lal B., Chartier P., Koenig J.F. (2000) Electrochim. Acta. 45:1911
Acknowledgments
The authors thank the Shanxi Natural Science Foundation (grants: 20041017) and Shanxi Scientific & Technological Promoted Project of China (grants: 031099) for the financial support of this work.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, YZ., Zhao, YX., Gao, CG. et al. Preparation and catalytic performance of Co3O4 catalysts for low-temperature CO oxidation. Catal Lett 116, 136–142 (2007). https://doi.org/10.1007/s10562-007-9099-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-007-9099-4