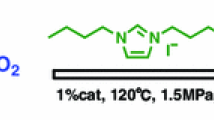
Abstract
One kind of novel ionic liquids (ILs) with a tertiary amino moiety and a quaternary ammonium group were synthesized and identified by FT-IR, 1H and 13C NMR. The elemental chemical state and basicity of ILs were determined by XPS and Hammett indicator method, respectively. Then the catalytic performance of these bi-functional catalysts was investigated in one-step synthesis of dimethyl carbonate (DMC) from ethylene oxide (EO), carbon dioxide and methanol. The best catalytic performance with 99% EO conversion and a maximum of 74% DMC selectivity was obtained using [N111,6N11]I as catalyst under optimized reaction conditions. And the catalyst could be reused for several times. Normally, stronger basicity could be obtained by altering the anions with different nucleophilicity in ILs and a better catalytic activity could be achieved correspondingly. A mechanism that both the ring opening of epoxide through nucleophilic attacks and the transesterification play an important role in the reaction was proposed based on experimental results.
Graphical Abstract
One kind of novel quaternary ammonium ionic liquids has been developed and for the first time used as bi-functional catalysts in the one-step synthesis of dimethyl carbonate (DMC) from ethylene oxide, CO2 and methanol. Normally, stronger basicity could be obtained by altering anions with different nucleophilicity in ILs and a better catalytic activity could be achieved correspondingly.












Similar content being viewed by others
References
Ono Y (1997) Appl Catal A 155:133
Tundo P, Selva M (2002) Acc Chem Res 35:706
Keller N, Rembmann G, Keller V (2010) J Mol Catal A 317:1
Pacheco MA, Marshall CL (1997) Energy Fuels 11:2
Shaikh AG, Sivaram S (1996) Chem Rev 96:951
Wei T, Wang MH, Wei W, Sun YH, Zhong B (2003) Green Chem 5:343
Babad H, Zeiler AG (1973) Chem Rev 73:75
King ST (1996) J Catal 161:530
Yamaguchi K, Ebitani K, Yoshida T, Yoshida H, Kaneda K (1999) J Am Chem Soc 121:4526
He JL, Wu TB, Zhang ZF, Ding KL, Han BX, Xie Y, Jiang T, Liu ZM (2007) Chem Eur J 13:6992
Sun J, Cheng WG, Fan W, Wang YH, Meng ZY, Zhang SJ (2009) Catal Today 50:423
Tian JS, Miao CX, Wang JQ, Cai F, Du Y, Zhao Y, He LN (2007) Green Chem 9:566
Wang MH, Wang H, Zhao H, Wei W, Sun YH (2007) Ind Eng Chem Res 46:2683
Fottinger K, Schlogl R, Rupprechter G (2008) Chem Commun 320
Sakakura T, Choi JC, Yasuda H (2007) Chem Rev 107:2365
Mikkelsen M, Jorgensen M, Krebs FC (2010) Energy Environ Sci 3:43
Bhanage BM, Fujita S, Ikushima Y, Arai M (2001) Appl Catal A 219:259
Bhanage BM, Fujita S, Ikushima Y, Torii K, Arai M (2003) Green Chem 5:71
Cui HY, Wang T, Wang FJ, Gu CR, Wang PL, Dai YY (2003) Ind Eng Chem Res 42:3865
Cui HY, Wang T, Wang FJ, Gu CR, Wang PL, Dai YY (2004) Ind Eng Chem Res 43:7732
Chang YH, Jiang T, Han BX, Liu ZM, Wu WZ, Gao L, Li JC, Gao HX, Zhao GY, Huang J (2004) Appl Catal A 263:179
Jiang Q, Yang Y (2004) Catal Lett 95:127
Kishimoto Y, Ogawa I (2004) Ind Eng Chem Res 43:8155
Li Y, Zhao XQ, Wang YJ (2005) Appl Catal A 279:205
Tian JS, Wang JQ, Chen JY, Fan JG, Cai F, He LN (2006) Appl Catal A 301:215
De CY, Lu B, Lv H, Yu YY, Bai Y, Cai QH (2009) Catal Lett 128:459
Wasserscheid P, Keim W (2000) Angew Chem Int Ed 39:3772
Welton T (2004) Coord Chem Rev 248:2459
Rodriguez H, Rogers RD (2010) Fluid Phase Equilib 294:7
Parvulescu VI, Hardacre C (2007) Chem Rev 107:2615
Zhang ZF, Xie Y, Li WJ, Hu SQ, Song JL, Jiang T, Han BX (2008) Angew Chem Int Ed 47:1127
Li XL, Ma XY, Shi F, Deng YQ (2010) ChemsusChem 3:71
Schneider HJ, Wang MX (1994) J Org Chem 59:7473
Jewett DM, Kilbourn MR (2002) J Labelled Compd Radiopharm 45:281
Holbrey JD, Seddon KR (1999) J Chem Soc Dalton Trans 2133
Myers C, Pennline H, Luebke D, Ilconich J, Dixon JK, Maginn EJ, Brennecke JF (2008) J Membr Sci 322:28
Kawanami H, Sasaki A, Matsui K, Ikushima Y (2003) Chem Commun 896
Calo V, Nacci A, Monopoli A, Fanizzi A (2002) Org Lett 4:2561
Anderson JL, Ding R, Armstrong DW (2005) J Am Chem Soc 127:593
Aggarwal A, Lancaster NL, Welton T (2002) Green Chem 4:517
Song JL, Zhang ZF, Han BX, Hui SQ, Li WJ, Xie Y (2008) Green Chem 10:1337
Sun J, Zhang SJ, Cheng WG, Ren JY (2008) Tetrahedron Lett 49:3588
Thomazeau C, Bourbigou HO, Magna L, Luts LS, Gilbert B (2003) J Am Chem Soc 125:5264
Bouby M, Billard I, Duplatre G, Simonin JP, Bernard O, Brunette JP, Grandmont GG (1999) Phys Chem Chem Phys 1:3765
Gu YL, Zhang J, Duan ZY, Deng YQ (2005) Adv Synth Catal 347:512
Acknowledgment
This work was financially supported by the National Natural Science Foundation of China (20533080).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, J., Wang, L., Shi, F. et al. Quaternary Ammonium Ionic Liquids as Bi-functional Catalysts for One-step Synthesis of Dimethyl Carbonate from Ethylene Oxide, Carbon Dioxide and Methanol. Catal Lett 141, 339–346 (2011). https://doi.org/10.1007/s10562-010-0498-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-010-0498-6