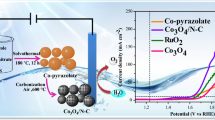
Abstract
The advanced oxygen evolution catalysts in alkaline solution play a growing role in alternative energy devices due to the need for clean and sustainable energy. In this paper, we report the cobalt phosphate nanoparticles embedded in N-doped carbon (Co3(PO4)2@N-C) using N,N′-piperazinebis (methylene-phosphonic acid) as both phosphate and carbon sources by two-step, hydrothermal method. The prepared Co3(PO4)2@N-C annealed at 600 °C exhibits advanced OER performance, with a current density of 10 mA cm−2 at a lower overpotential of 290 mV, a Tafel slope of 82 mV dec−1 and superior durability in 1.0 M KOH solution. This kind of material with MOF as precursor has wide application prospect in electro-chemistry field, especially for OER.
Graphical Abstract




Similar content being viewed by others
References
Zou XX et al (2013) Efficient oxygen evolution reaction catalyzed by low-density Ni-doped Co3O4 nanomaterials derived from metal-embedded graphitic C3N4. Chem Commun 49(68):7522–7524
Landon J et al (2012) Spectroscopic characterization of mixed Fe–Ni oxide electrocatalysts for the oxygen evolution reaction in alkaline electrolytes. ACS Catal 2(8):1793–1801
Najafpour MM et al (2012) Nano-sized manganese oxide-bovine serum albumin was synthesized and characterized. It is promising and biomimetic catalyst for water oxidation. RSC Adv 2(30):11253–11257
Chen S et al (2013) Three-dimensional N-doped graphene hydrogel/NiCo double hydroxide electrocatalysts for highly efficient oxygen evolution. Angew Chem Int Ed 52(51):13567–13570
Kanan MW, Nocera DG (2008) In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 321(5892):1072–1075
Han L, Dong SJ, Wang EK (2016) Transition-metal (Co, Ni, and Fe)-based electrocatalysts for the water oxidation reaction. Adv Mater 28(42):9266–9291
Chaikittisilp W et al (2014) Synthesis of nanoporous carbon–cobalt–oxide hybrid electrocatalysts by thermal conversion of metal–organic frameworks. Chemistry 20(15):4217–4221
Gascon J et al (2014) Metal organic framework catalysis: Quo vadis? ACS Catal 4(2):361–378
Xia W et al (2015) Metal-organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ Sci 8(7):1837–1866
Xu X et al (2012) Spindle-like mesoporous alpha-Fe2O3 anode material prepared from MOF template for high-rate lithium batteries. Nano Lett 12(9):4988–4991
Zhao SL et al (2014) Carbonized nanoscale metal–organic frameworks as high performance electrocatalyst for oxygen reduction reaction. ACS Nano 8(12):12660–12668
Wang H et al (2014) Preparation, characterization and bifunctional catalytic properties of MOF(Fe/Co) catalyst for oxygen reduction/evolution reactions in alkaline electrolyte. Int J Hydrog Energy 39(28):16179–16186
Shang NZ et al (2016) Ag/Pd nanoparticles supported on amine-functionalized metal–organic framework for catalytic hydrolysis of ammonia borane. Int J Hydrog Energy 41(2):944–950
Dou S et al (2016) Etched and doped Co9S8/graphene hybrid for oxygen electrocatalysis. Energy Environ Sci 9(4):1320–1326
Yu HY et al (2016) Cu, N-codoped hierarchical porous carbons as electrocatalysts for oxygen reduction reaction. ACS Appl Mater Interfaces 8(33):21431–21439
Ma TY et al (2016) Interacting carbon nitride and titanium carbide nanosheets for high-performance oxygen evolution. Angew Chem Int Ed 55(3):1138–1142
Liu YY et al (2016) Transition metals (Fe, Co, and Ni) encapsulated in nitrogen-doped carbon nanotubes as bi-functional catalysts for oxygen electrode reactions. J Mater Chem A 4(5):1694–1701
Ma TY et al (2014) Metal–organic framework derived hybrid Co3O4-carbon porous nanowire arrays as reversible oxygen evolution electrodes. J Am Chem Soc 136(39):13925–13931
Long JY, Gong Y, Lin JH (2017) Metal–organic framework-derived Co9S8@CoS@CoO@C nanoparticles as efficient electro- and photo-catalysts for the oxygen evolution reaction. J Mater Chem A 5(21):10495–10509
Bendi R et al (2016) Metal organic framework-derived metal phosphates as electrode materials for supercapacitors. Adv Energy Mater 6(3)
Li XY et al (2016) ZIF-67-derived Co-NC@CoP-NC nanopolyhedra as an efficient bifunctional oxygen electrocatalyst. J Mater Chem A 4(41):15836–15840
Liu YR et al (2016) Novel CoP hollow prisms as bifunctional electrocatalysts for hydrogen evolution reaction in acid media and overall water-splitting in basic media. Electrochim Acta 220:98–106
Pu ZH et al (2017) General strategy for the synthesis of transition-metal phosphide/N-doped carbon frameworks for hydrogen and oxygen evolution. ACS Appl Mater Interfaces 9(19):16187–16193
Chang JF et al (2015) Surface oxidized cobalt-phosphide nanorods as an advanced oxygen evolution catalyst in alkaline solution. ACS Catal 5(11):6874–6878
Surendranath Y, Kanan MW, Nocera DG (2010) Mechanistic studies of the oxygen evolution reaction by a cobalt-phosphate catalyst at neutral pH. J Am Chem Soc 132(46):16501–16509
Ai GJ et al (2015) Cobalt phosphate modified TiO2 nanowire arrays as co-catalysts for solar water splitting. Nanoscale 7(15):6722–6728
Alhendawi H et al (2013) A new layered zirconium biphosphonate framework covalently pillared with N,N′-piperazinebis(methylene) moiety: synthesis and characterization. J Porous Mater 20(5):1189–1194
Zhou W et al (2011) Amorphous iron oxide decorated 3D heterostructured electrode for highly efficient oxygen reduction. Chem Mater 23(18):4193–4198
Bergmann A et al (2015) Reversible amorphization and the catalytically active state of crystalline Co3O4 during oxygen evolution. Nat Commun 6:8625
Zhao J et al (2014) Self-template construction of hollow Co3O4 microspheres from porous ultrathin nanosheets and efficient noble metal-free water oxidation catalysts. Nanoscale 6(13):7255–7262
Chen ZY et al (2017) Ag-enhanced catalytic performance of ordered mesoporous Fe–N-graphitic carbons for oxygen electroreduction. Catal Lett 147(11):2745–2754
Yang J et al (2010) Synthesis and characterization of cobalt hydroxide, cobalt oxyhydroxide, and cobalt oxide nanodiscs. J Phys Chem C 114(1):111–119
Xing M et al (2014) Cobalt vanadate as highly active, stable, noble metal-free oxygen evolution electrocatalyst. J Mater Chem A 2(43):18435–18443
Hu GR et al (2008) Comparison of AlPO4- and Co3(PO4)(2)-coated LiNi0.8Co0.2O2 cathode materials for Li-ion battery. Electrochim Acta 53(5):2567–2573
Yuan CZ et al (2016) Cobalt phosphate nanoparticles decorated with nitrogen-doped carbon layers as highly active and stable electrocatalysts for the oxygen evolution reaction. J Mater Chem A 4(21):8155–8160
Zhang CZ et al (2013) Synthesis of amino-functionalized graphene as metal-free catalyst and exploration of the roles of various nitrogen states in oxygen reduction reaction. Nano Energy 2(1):88–97
Nagaiah TC et al (2012) Mesoporous nitrogen-rich carbon materials as catalysts for the oxygen reduction reaction in alkaline solution. ChemSusChem 5(4):637–641
Shruthi TK et al (2014) Functionalization of graphene with nitrogen using ethylenediaminetetraacetic acid and their electrochemical energy storage properties. RSC Adv 4(46):24248–24255
Li XZ et al (2015) MOF derived Co3O4 nanoparticles embedded in N-doped mesoporous carbon layer/MWCNT hybrids: extraordinary bi-functional electrocatalysts for OER and ORR. J Mater Chem A 3(33):17392–17402
Zhou WJ et al (2015) N-doped carbon-wrapped cobalt nanoparticles on N-doped graphene nanosheets for high-efficiency hydrogen production. Chem Mater 27(6):2026–2032
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Feng, P., Cheng, X., Li, J. et al. Co3(PO4)2 Nanoparticles Embedded in Nitrogen-Doped Carbon as an Advanced Electrocatalyst for OER in Alkaline Solution. Catal Lett 148, 214–219 (2018). https://doi.org/10.1007/s10562-017-2251-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-017-2251-x