Abstract
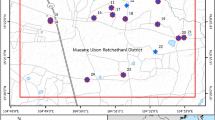
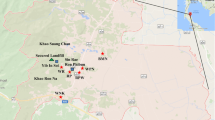
Arsenic bioavailability in rock, soil and water resources is notoriously hazardous. Geogenic arsenic enters the body and adversely affects many biochemical processes in animals and humans, posing risk to public health. Chelpu is located in NE Iran, where realgar, orpiment and pyrite mineralization is the source of arsenic in the macroenvironment. Using cluster random sampling strategy eight rocks, 23 soils, 12 drinking water resources, 36 human urine and hair samples and 15 adult sheep urine and wool samples in several large-scale herds in the area were randomly taken for quantification of arsenic in rock/soil/water, wool/hair/urine. Arsenic levels in rock/soil/water and wool/hair/urine were measured using inductively coupled plasma spectroscopy and atomic absorption spectrophotometry, respectively. While arsenic levels in rocks, soils and water resources hazardously ranged 9.40–25,873.3 mg kg−1, 7.10–1448.80 mg kg−1 and 12–606 μg L−1, respectively, arsenic concentrations in humans’ hair and urine and sheep’s wool and urine varied from 0.37–1.37 μg g−1 and 9–271.4 μg L−1 and 0.3–3.11 μg g−1 and 29.1–1015 μg L−1, respectively. Local sheep and human were widely sick and slightly anemic. Hematological examination of the inhabitants revealed that geogenic arsenic could harm blood cells, potentially resulting in many other hematoimmunological disorders including cancer. The findings warn widespread exposure of animals and human in this agroecologically and geopolitically important region (i.e., its proximity with Afghanistan, Pakistan and Turkmenistan) and give a clue on how arsenic could induce infectious and non-infectious diseases in highly exposed human/animals.




Similar content being viewed by others
References
Andrew, A. S., Jewell, D. A., Mason, R. A., Whitfield, M. L., Moore, J. H., & Karagas, M. R. (2008). Drinking-water arsenic exposure modulates gene expression in human lymphocytes from a U.S. population. Environmental Health Perspectives, 116(4), 524–531.
Argos, M., Kibriya, M. G., Parvez, F., Jasmine, F., Rakibuz-Zaman, M., & Ahsan, H. (2006). Gene expression profiles in peripheral lymphocytes by arsenic exposure and skin lesion status in a Bangladeshi population. Cancer Epidemiology Biomarkers and Prevention, 15(7), 1367–1375.
ATSDR. (2007). Agency for toxic substances and disease registry. ToxGuide For Arsenic.
Banerjee, N., Banerjee, S., Sen, R., Bandyopadhyay, A., Sarma, N., Majumder, P., et al. (2009). Chronic arsenic exposure impairs macrophage functions in the exposed individuals. Journal of Clinical Immunology, 29(5), 582–594.
Binet, F., Cavalli, H., Moisan, E., & Girard, E. (2005). Arsenic trioxide (AT) is a novel human neutrophil pro-apoptotic agent: effects of catalase on AT-induced apoptosis, degradation of cytoskeletal proteins and de novo protein synthesis. British Journal of Haematology, 132(3), 349–358.
Binet, F., & Girard, E. (2008). Novel human neutrophil agonistic properties of arsenic trioxide: involvement of p38 mitogen-activated protein kinase and/or c-jun NH2-terminal MAPK but not extracellular signal-regulated kinases-1/2. Journal of Leukocyte Biology, 84(6), 1613–1622.
Biswas, D., Banerjee, M., Sen, Gargi, Das, J. K., Banerjee, A., Su, T. J., et al. (2008). Mechanism of erythrocyte death in human population exposed to arsenic through drinking water. Toxicology and Applied Pharmacology, 230(1), 57–66.
Colin-Torres, C. G., Murillo-Jimenez, J. M., Del Razo, L. M., Sanchez-Pena, L., Becerra-Rueda, O. F., & Marmolejo-Rodriguez, A. J. (2014). Urinary arsenic levels influenced by abandoned mine tailings in the Southernmost Baja California Peninsula, Mexico. Environmental Geochemistry and Health, 36(5), 845–854.
Das, D., Chatterjee, A., Mandal, B. K., Samanta, G., Chakraborti, D., & Chanda, B. (1995). Arsenic in ground water in six districts of West bengal, India: the biggest arsenic calamity in the world. Part 2. Arsenic concentration in drinking water, hair, nails, urine, skin-scale and liver tissue (biopsy) of the affected people. Analyst, 120(3), 917–924.
de la Fuente, H., Portales-Pérez, D., Baranda, L., Díaz-Barriga, F., Saavedra-Alanís, V., Layseca, E., & González-Amaro, R. (2002). Effects of arsenic, cadmium and lead on the induction of apoptosis of normal human mononuclear cells. Clinical and Experimental Immunology, 129(1), 69–77.
Duker, A. A., Carranza, E. J., & Hale, M. (2005). Arsenic geochemistry and health. Environment International, 31(5), 631–641.
Fillol, C., Dor, F., Labat, L., Boltz, L., Bouard, J. L., Mantey, K., et al. (2010). Urinary arsenic concentration and speciation in residents living in an area with naturally contaminated soils. Science of the Total Environment, 408(5), 1190–1194.
Gerdsen, R., Stockfleth, E., Uerlich, M., Fartasch, M., Steen, K. H., & Bieber, T. (2000). Papular palmoplantar hyperkeratosis following chronic medical exposure to arsenic: Human papillomavirus as a co-factor in the pathogenesis of arsenical keratosis? Acta Dermato-Venereologica, 80(4), 292–293.
Hall, M., Chen, Y., Ahsan, H., Slavkovich, V., Geen, A. V., Parvez, F., & Graziano, J. (2006). Blood arsenic as a biomarker of arsenic exposure: Result from a prospective study. Toxicology, 225(2–3), 225–233.
Hambidge, K. M. (1982). Hair analyses: worthless for vitamins, limited for minerals. American Journal of Clinical Nutrition, 36(5), 943–949.
Hughes, M. (2002). Arsenic toxicity and potential mechanisms of action. Toxicology Letters, 133(1), 1–16.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. (2004). Some drinking-water disinfectants and contaminants, including arsenic: IARC working group on the evaluation of carcinogenic risks to humans (Vol. 84). Lyon: IARC Press.
Kyle, R. A., & Pearse, G. L. (1965). Haematological aspects of arsenic intoxication. New England Journal of Medicine, 273, 18–23.
Li, S., Xiao, T., & Zheng, B. (2012). Medical geology of arsenic, selenium and thallium in China. Science of the Total Environment, 421–422, 31–40.
Luna, A. L., Acosta-Saavedra, L. C., Lopez-Carrillo, L., Conde, P., Vera, E., Vizcaya-aruiz, A. D., et al. (2010). Arsenic alters monocyte superoxide anion and nitric oxide production in environmentally exposed children. Toxicology and Applied Pharmacology, 245(2), 244–251.
Mehrzad, J. (2012). Molecular aspects of neutrophils as pivotal circulating cellular innate immune systems to protect mammary gland from pathogens. In J. R. Kanwar (Ed.), Recent advances in immunology to target cancer, inflammation and infections (pp. 383–422). Rijeka, Croatia: InTech.
Mehrzad, J., Dosogne, H., Meyer, E., Heyneman, R., & Burvenich, C. (2001). Respiratory burst activity of blood and milk neutrophils in dairy cows during different stages of lactation. Journal of Dairy Research, 68(3), 399–415.
Mejia, J., Diaz-barriga, F., Calderon, J., Rios, C., & Jimenez-Capdeville, M. E. (1997). Effect of lead–arsenic combined exposure on central monoaminergic nervous systems. Neurotoxicology and Teratology, 19(6), 489–497.
Mohammadi, A., Mehrzad, J., Mahmoudi, M., & Schneider, M. (2014). Environmentally relevant level of aflatoxin B1 dysregulates human dendritic cells through signaling on key toll-like receptors. International Journal of Toxicology, 33(3), 175–186.
Morzadec, C., Bouezzedine, F., Macoch, M., Fardel, O., & Vernhet, L. (2012). Inorganic arsenic impairs proliferation and cytokine expression in human primary T lymphocytes. Toxicology, 300(1–2), 46–56.
National Institute for Research and safety for the prevention of occupational accidents and diseases (INRS). (2008). Biotox database, http://www.inrs.fr/biotox.
Pimparkar, B. D., & Bhave, A. (2010). Arsenicosis: review of recent advances. Journal of the Association of Physicians of India, 58(24), 617–629.
Porter, K. E., Basu, A., Hubbard, A. E., Bates, M. N., Kalman, D., Rey, O., et al. (2010). Association of genetic variation in cystathionine-beta-synthase and arsenic metabolism. Environmental Research, 110(6), 580–587.
Qiu, L., Song, Z., & Setaluri, V. (2014). Oxidative stress and vitiligo: The Nrf2-ARE signaling connection. Journal of Investigative Dermatology, 134(8), 2074–2076.
Ruiz-Ramos, R., Lopez-Carrillo, L., Rios-Perez, A. D., Vizcaya-Ruíz, A., & Cebrian, M. E. (2009). Sodium arsenite induces ROS generation, DNA oxidative damage, HO-1 and c-Myc proteins, NF-kappaB activation and cell proliferation in human breast cancer MCF-7 cells. Mutation Research, 674(1–2), 109–115.
Schulz, H., Nagymajtenyi, L., Institoris, L., Papp, A., & Siroki, O. (2002). A study on behavioral, neurotoxicological, and immunotoxicological effects of subchronic arsenic treatment in rats. Journal of Toxicology and Environmental Health Part A, 65(16), 1181–1193.
Schwartz, J., Landrigan, P. J., Baker, E. L., Orenstein, W. A., & Lindern, I. H. (1990). Lead-induced anemia: Dose-response relationships and evidence for a threshold. American Journal of Public Health, 80(2), 165–168.
Sikder, M. S., Shakya, N. B., Rahman, M. H., & Ansari, N. P. (2008). Association between anemia and grading of arsenicosis. Journal of Pakistan Association of Dermatologists, 18(4), 202–206.
Silva, A. M., Novelli, E. L., Fascineli, M. L., & Almeida, J. A. (1999). Impact of an environmentally realistic intake of water contaminant and superoxide formation on tissues of rats. Environmental Pollution, 105(2), 243–249.
Smedley, P. L., & Kinniburgh, D. G. (2002). A review of the source, behaviour and distribution of arsenic in natural water. Applied Geochemistry, 17(5), 517–568.
Steinmaus, C., Ferreccio, C., Romo, J. A., Yuan, Y., Cortes, S., Marshall, G., et al. (2013). Drinking water arsenic in northern chile: High cancer risks 40 years after exposure cessation. Cancer Epidemiology Biomarkers and Prevention, 22(4), 623–630.
Steinmaus, C., Ferreccio, C., Yuan, Y., Acevedo, J., González, F., Perez, L., et al. (2014). Elevated lung cancer in younger adults and low concentrations of arsenic in water. American Journal of Epidemiology, 180(11), 1082–1087.
Steinmaus, C., Yuan, Y., Kalman, D., Rey, O. A., Skibola, C. F., Dauphine, D., et al. (2010). Individual differences in arsenic metabolism and lung cancer in a case–control study in Cordoba, Argentina. Toxicology and Applied Pharmacology, 247(2), 138–145.
Tamasi, G., & Cini, R. (2004). Heavy metals in drinking waters from Mount Amiata (Tuscany, Italy). Possible risks from arsenic for public health in the province of Siena. Science of the Total Environment, 327(1–3), 41–51.
Taylor, S. R. (1964). Abundance of chemical elements in the continental crust: a new table. Geochemica et Cosmochimica Acta, 28(8), 1273–1285.
Turekian, K. K., & Wedepohl, K. H. (1961). Distribution of the elements in some major units of the earth’s crust. Geological Society of America Bulletin, 72(2), 175–192.
U.S. Agency for Toxic Substances and Disease Registry (US ATSDR), Toxicological profile for Arsenic, Atlanta, GA. (2007). http://www.atsdr.cdc.gov/toxprofiles/tp2.pdf.
U.S. Environmental Protection Agency Office of Solid Waste and Emergency Response. (1983). Hazardous waste land treatment, SW-874 (pp. 273).
Vizcaya-Ruiz, A. D., Barbier, O., Ruiz-Ramos, R., & Cebrian, M. E. (2009). Biomarkers of oxidative stress and damage in human populations exposed to arsenic. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 674(1–2), 85–92.
Wilson, C. S., Lockwood, V. P., Ashley, P. M., & Tighe, M. (2010). The chemistry and behavior of antimony in the soil environment with comparisons to arsenic: A critical review. Environmental Pollution, 158(5), 1169–1181.
Woods, J. S., & Fowler, B. A. (1978). Altered regulation of mammalian hepatic heme biosynthesis and urinary porphy excretion during prolonged exposure to sodium arsenate. Toxicology and Applied Pharmacology, 43(2), 361–371.
World Health Organization. (2011a). Guidelines for drinking-water quality.
World Health Organization. (2011b). Hemoglobin concentrations for the diagnosis of anaemia and assessment of severity.
Wu, M. M., Chiou, H. Y., Wang, T. W., Hsueh, Y. M., Wang, I. H., & Chen, C. J. (2001). Association of blood arsenic levels with increased reactive oxidants and decreased antioxidant capacity in human population of northeastern Taiwan. Environmental Health Perspectives, 109(10), 1011–1017.
Acknowledgments
This study has been supported by Ferdowsi University of Mashhad. The authors wish to thank Prof. Dr. Daniel Holm and Ms. Yasaman Rafihgdoust for the water samples analysis in Kent State University, Geology Department. The study was from two MSc thesis, first (M.T.) and sixth (Sh. H) authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standard
The manuscript does not contain clinical studies or specified patient data.
Rights and permissions
About this article
Cite this article
Taheri, M., Mehrzad, J., Mahmudy Gharaie, M.H. et al. High soil and groundwater arsenic levels induce high body arsenic loads, health risk and potential anemia for inhabitants of northeastern Iran. Environ Geochem Health 38, 469–482 (2016). https://doi.org/10.1007/s10653-015-9733-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-015-9733-9