Abstract
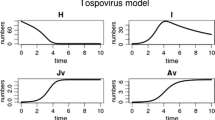
Natural enemies have long been used in biological control programs to mitigate the damage caused by herbivory. Many herbivorous insect species also act as plant virus vectors, enabling virus transmission from plant to plant and hence disease development in a plant population. Whilst an intuitive assumption would be to expect a decrease in vector numbers to lead to subsequent reductions in virus transmission, recent evidence suggests that introduction of natural enemies (parasitoids and predators) may in some cases increase plant virus transmission while at the same time reducing vector numbers. In this paper we review the evidence for plant-virus-vector-natural enemy interactions, the signalling mechanisms involved and their implications for virus transmission, and show how a modelling approach can assist in identifying the key parameters and relationships involved in determining the disease outcome. A mathematical model linking the population dynamics of a vector-parasitoid system with virus transmission was used to investigate the effects of virus inoculation and acquisition rates, parasitoid attack rate and vector aggregation on disease dynamics across a wide range of parameter value combinations. Virus spread was found to increase with enhanced inoculation, acquisition and parasitoid attack rate but decrease with high levels of vector aggregation.






Similar content being viewed by others
References
Abrams, P. A. (1987). Indirect interactions between species that share a predator: varieties on a theme. In W. C. Kerfoot & A. Sih (Eds.), Predation: direct and indirect impacts on aquatic communities (pp. 38–54). New England: Univ. Press.
Alvarez, A. E., Garzo, E., Verbeek, M., Vosman, B., Dicke, M., & Tjallingii, W. F. (2007). Infection of potato plants with potato leafroll virus changes attraction and feeding behaviour of Myzus persicae. Entomologia Experimentalis et Applicata, 125, 135–144.
Arimura, G., Matsui, K., & Takabayashi, J. (2009). Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiology, 50, 911–923.
Bailey, S. M., Irwin, M. E., Kampmeier, G. E., Eastman, C. E., & Hewings, A. D. (1995). Physical and biological perturbations—their effect on the movement of apterous Rhopalosiphon padi (Homoptera. Aphididae) and localised spread of barley yellow dwarf virus. Environmental Entomology, 24, 24–33.
Baldwin, I. T., Halitschke, R., Pascholde, A., von Dahl, C. C., & Preston, C. A. (2006). Volatile signalling in plant-plant interactions: “Talking trees” in the genomics era. Science, 311, 812–815.
Bowers, W. S., Nault, L. R., Webb, R. E., & Dutky, S. R. (1972). Aphid alarm pheromones. Science, 177, 1121–1122.
Braendle, C., & Weisser, W. W. (2001). Variation in escape behaviour of red and green clones of the pea aphid. Journal of Insect Behaviour, 14, 497–509.
Busgen, M. (1891). Biologische Studien an Pflanzen und Pflanzenlausen. Wissenschaftliche Zeitschrift. Naturwiss. Reihe/ Friedriche-Schiller-Univ. Jena, 25, 339–428.
Castle, S. J., & Berger, P. H. (1993). Rates of growth and increase of M. persicae on virus-infected potatoes according to type of virus-vector relationship. Entomologia Experimentalis et Applicata, 69, 51–60.
Castle, S. J., Mowry, T. M., & Berger, P. H. (1998). Differential settling by Myzus persicae (Homoptera: Aphididae) on various virus-infected host plants. Annals of the Entomological Society of America, 91, 661–667.
Chase, J. M. (1999). To grow or to reproduce? The role of life-history plasticity in food web dynamics. American Naturalist, 154, 571–586.
Chen, Z. (2009). Do natural enemies affect the incidences of vector-transmitted plant diseases? A theoretical investigation. MRes thesis, Imperial College London, 44 pp.
Chen, Z., & Jeger, M. J. (2012). Comparing the effects of natural enemies on plant virus epidemiology: three models from different perspectives. In K. Stevenson & M. J. Jeger (Eds.), Exercises in plant disease epidemiology (2nd ed.). St Paul: APS Press. in press.
Davis, J. J. (1914). The yellow clover aphis (Callipterus trifolii Monell.). Technical Series U.S. Bureau of Entomology, 25, 17–40.
de Vos, M., & Jander, G. (2010). Volatile communication in plant-aphid interactions. Current Opinion in Plant Biology, 13, 366–371.
Dicke, M. (2009). Behavioural and community ecology of plants that cry for help. Plant, Cell and Environment, 32, 654–665.
Dicke, M., & Baldwin, I. T. (2010). The evolutionary context for herbivore induced plant volatiles: beyond the ‘cry for help’. Trends in Plant Science, 15, 167–175.
Dicke, M., Sabelis, M. W., & Vandenberg, H. (1989). Does the prey preference change as a result of prey species being presented together—analysis of prey selection by the predatory mite Typhlodromus pyri (Acarina, Phytoseiidae). Oecologia, 81, 302–309.
Dicke, M., Vanbaarlen, P., Wessels, R., & Dijkman, H. (1993). Herbivore induces systemic production of plant volatiles that attract predators of the herbivore—extraction of endogenous elicitors. Journal of Chemical Ecology, 19, 581–599.
Dill, L. M., Fraser, A. H. G., & Roitberg, B. D. (1990). The economics of escape behaviour in the pea aphid, Acyrthosiphon pisum. Oecologia, 83, 473–478.
Donaldson, J. R., & Gratton, C. (2007). Antagonistic effects of soybean viruses on soybean aphid performance. Environmental Entomology, 36, 918–925.
Doring, T. F., & Chittka, L. (2006). Visual ecology of aphids-a critical review of the role of colours in host finding. Arthropod Plant Interactions, 1, 3–16.
Du, Y., Poppy, G. M., Powell, W., Pickett, J. A., Wadhams, L. J., & Woodcock, C. M. (1998). Identification of semiochemicals released during aphid feeding that attract the parasitoid Aphidius ervi. Journal of Chemical Ecology, 24, 1355–1368.
Edwards, L. J., Siddall, J. B., Dunham, L. L., Uden, P., & Kislow, C. J. (1973). Trans-β-farnesene, alarm pheromone of the green peach aphid, Myzus persicae (Sulzer). Nature, 241, 126–127.
Eigenbrode, S. D., Ding, H., Shiel, P., & Berger, P. H. (2002). Volatiles from potato plants infected with potato leafroll virus attract and arrest the virus vector Myzus persicae (Homoptera: Aphididae). Proceedings of the Royal Society B-Biological Sciences, 269, 455–460.
Evans, D. L., & Schmidt, J. O. (1990). Insect defenses: Adaptive mechanisms and strategies of prey and predators. Albany: State University of New York Press.
Fereres, A., Shukle, R. H., Araya, J. E., & Foster, J. E. (1990). Probing and feeding-behaviour of Sitobion-avenae F (Hom Aphididae) on 3 wheat cultivars infected with barley yellow dwarf virus. Journal of Applied Entomology, 109, 29–36.
Fereres, A., Kampmeier, G. E., & Irwin, M. E. (1999). Aphid attraction and preference for soybean and pepper plants infected with potyviridae. Annals of the Entomological Society of America, 92, 543–548.
Fernandes, G. W., Ribeiro, S. P., & Pimenta, H. R. (1994). Herbivory by chewing and sucking insects on Tabebuia ochracea. Biotropica, 26, 302–207.
Fiebig, M., Poehling, H. M., & Borgemeister, C. (2003). Barley yellow dwarf virus, wheat, and Sitobion avenae: a case of trilateral interactions. Entomologia Experimentalis et Applicata, 110, 11–21.
Fievet, V., Le Guigo, P., Casquet, J., Poinsot, D., & Outreman, Y. (2009). Living with the dead: when the body count rises, prey stick around. Behavioural Ecology, 20, 251–257.
Francis, F., Lognay, G., & Haubruge, E. (2003). Olfactory responses to aphid and host plant volatile releases: (E)-β-farnesene an effective kairomone for the predator Adalia bipunctata. Journal of Chemical Ecology, 30, 741–755.
Fritzsche-Hoballah, M. E., & Turlings, T. C. J. (2001). Response of natural populations of predators and parasitoid to artificially induce volatiles emissions in maize plants (Zea mays L.). Agricultural and Forest Entomology, 3, 201–209.
Froissart, R., Doumayrou, J., Vuillaume, F., Alizon, S., & Michalakis, Y. (2010). The virulence-transmission trade-off in vector-borne plant viruses: a review of (non-)existing studies. Philosophical Transactions of the Royal Society B-Biological Sciences, 365, 1907–1918.
Garrett, K. A., Dendy, S. P., Frank, E. E., Rouse, M. N., & Tracers, S. E. (2006). Climate change effects on plant disease: genomes to ecosystems. Annual Review of Phytopathology, 44, 489–509.
Geervliet, J. B. F., Vet, L. E. M., & Dicke, M. (1994). Volaties from damaged plants as major cues in long-range host-searching by the specialist parasitoid Cotesia rubecula. Entomologia Experimentalis et Applicata, 73, 289–297.
Geervliet, J. B. F., Vet, L. E. M., & Dicke, M. (1996). Innate responses of the parasitoids Cotesia glomerata and C rubecula (Hymenoptera: Braconidae) to volatiles from different plant-herbivore complexes. Journal of Insect Behaviour, 9, 525–538.
Girling, R. D., Madison, R., Hassall, M., Poppy, G. M., & Turner, J. G. (2008). Investigations into plant biochemical wound-response pathways involved in the production of aphid-induced plant volatiles. Journal of Experimental Botany, 59, 3077–3085.
Gross, P. (1993). Insect behavioural and morphological defences against parasitoids. Annual Review of Entomology, 38, 251–73.
Guerrieri, E., Pennacchio, F., & Tremblay, E. (1993). Flight behaviour of the aphid parasitoid Aphidius ervi (Hymenoptera, Braconidae) in response to plant and host volatiles. European Journal of Entomology, 90(4), 15–421.
Guerrieri, E., Poppy, G. M., Powell, W., Tremblay, E., & Pennacchio, F. (1999). Induction and systemic release of herbivore induced plant volatiles mediating in-flight orientation of Aphidius ervi. Journal of Chemical Ecology, 6, 1247–1261.
Hodge, S., & Powell, G. (2008). Complex interactions between a plant pathogen and insect parasitoid via the shared vector-host: consequences for host plant infection. Oecologia, 157, 387–397.
Houston, A. I., McNamara, J. M., & Hutchinson, J. M. C. (1993). General results concerning the trade-off between gaining energy and avoiding predation. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences, 341, 375–397.
Itô, Y. (1989). The evolutionary biology of sterile soldiers in aphids. Trends in Ecology & Evolution, 4, 69–73.
Jeger, M. J., van den Bosch, F., Madden, L. V., & Holt, J. (1998). A model for analysing plant-virus transmission characteristics and epidemic development. IMA Journal of Mathematics Applied in Medicine and Biology, 15, 1–18.
Jeger, M. J., Holt, J., van den Bosch, F., & Madden, L. V. (2004). Epidemiology of insect-transmitted plant viruses: modelling disease dynamics and control interventions. Physiological Entomology, 29, 331–349.
Jeger, M. J., Chen, Z., Powell, G., Hodge, S., & van den Bosch, F. (2011). Interactions in a host plant-virus-vector-parasitoid system: modelling the consequences for virus transmission and disease dynamics. Virus Research (in press).
Jiu, M., Zhou, X., Tong, L., Xu, J., Yang, X., Wan, F., & Liu, S. (2007). Vector-virus mutualism accelerates population increase of an invasive whitefly. Plos One, 2, e182.
Kennedy, J. S. (1951). Benefits to aphids from feeding on galled and virus-infected leaves. Nature, 168, 825–826.
Kessler, A., & Halitschke, R. (2007). Specificity and complexity: the impact of herbivore induced plant responses on arthropod community structure. Current Opinion in Biology, 10, 409–414.
Kislow, C. J., & Edwards, L. J. (1972). Repellent odours in aphids. Nature, 235, 108–109.
Kunert, G., & Weisser, W. W. (2003). The interplay between density- and trait-mediated effects in predator–prey interactions: a case study in aphid wing polymorphism. Oecologia, 135, 304–312.
Kunert, G., & Weisser, W. W. (2008). The importance of antennae for pea aphid wing induction in the presence of natural enemies. Bulletin of Entomological Research, 95, 125–131.
Kunert, G., Otto, S., Rose, U. S. R., Gershenzon, J., & Weisser, W. W. (2005). Alarm pheromone mediates production of winged dispersal morphs in aphids. Ecology Letters, 8, 596–603.
Losey, J. E. (1996). Synergism between ground and foliar-foraging predators of aphids in alfalfa. PhD Dissertation, University of Maryland, U.S.A.
Losey, J. E., & Denno, R. F. (1998). Positive predator-predator interactions: enhanced predation rates and synergistic suppression of aphid populations. Ecological Society of America, 79, 2143–2152.
Madden, L. V., Nault, L. R., Murral, D. J., & Apelt, M. R. (1995). Spatial pattern analysis of the incidence of aster yellows disease in lettuce. Researches on Population Ecology, 37, 279–289.
Madden, L. V., Jeger, M. J., & van den Bosch, F. (2000). A theoretical assessment of the effects of vector-virus transmission mechanism on plant virus disease epidemics. Phytopathology, 90, 576–594.
Maris, P. C., Joosten, N. N., Goldbach, R. W., & Peters, D. (2004). Tomato spotted wilt virus infection improve host suitability for its vector Frankliniella occidentalis. Phytopathology, 94, 706–711.
Mauck, K. E., De Moraes, C. M., & Mescher, M. C. (2010). Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. PNAS, 107, 3600–3605.
McKenzie, C. L., Shatters, R. G., Jr., Doostdar, H., Lee, S. D., Inbar, M., & Mayer, R. T. (2002). Effect of geminivirus infection and Bemisia infestation on accumulation of pathogenesis-related proteins in tomato. Archives of Insect Biochemistry and Physiology, 49, 203–214.
Medina-Ortega, K. J., Bosque-Perez, N. A., Ngumbi, E., Jimenez-Martinez, E. S., & Eigenbrode, S. D. (2009). Rhopalosiphum padi (Hemiptera: Aphididae) responses to volatile cues from barley yellow dwarf virus-infected wheat. Environmental Entomology, 38, 836–845.
Meiners, T., & Hilker, M. (2000). Induction of plant synomones by oviposition of a phytophagous insects. Journal of Chemical Ecology, 26, 221–232.
Micha, S. G., & Wyss, U. (1995). The importance of plant odours for host searching of Aphidius uzbekistanicus (Hymenoptera, Aphididae), a parasitoid of the grain aphid (Sitobion avenae). Gesunde Pflanzen, 47, 300–307.
Mondor, E. B., & Roitberg, B. D. (2003). Age-dependent fitness costs of alarm signalling in aphids. Canadian Journal of Zoology—Revue Canadienne de Zoologie, 81, 757–762.
Montgomery, M. E., & Nault, L. R. (1977). Comparative response of aphids to alarm pheromone, (E)-β-farnesene. Entomologia Experimentalis et Applicata, 22, 236–242.
Musser, R. O., Hum-Musser, S. M., Felton, G. W., & Gergerich, R. C. (2003). Increased larval growth and preference for virus-infected leaves by the Mexican bean beetle, Epilachna varivestis Mulsant, a plant virus vector. Journal of Insect Behaviour, 16, 247–256.
Nault, L. R., Edwards, L. J., & Styer, W. E. (1973). Aphid alarm pheromones: secretion and reception. Environmental Entomology, 2, 101–105.
Niku, B. (1975). Behaviour and fecundity of apterous pea aphids (Acyrthosiphon pisum) after a drop-reaction. Entomologia Experimentalis et Applicata, 18, 17–30.
Pare, P. W., & Tumlinson, J. H. (1997). De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiology, 114, 1161–1167.
Peacor, S. D. (2003). Phenotypic modifications to conspecific density arising from predation risk assessment. Oikos, 100, 409–415.
Pickett, J. A., & Glinwood, R. T. (2007). Chemical ecology. In H. Van Emden & R. Harrington (Eds.), Aphids as crop pests (pp. 235–260). Wallingford: CAB.
Pickett, J. A., & Griffiths, D. C. (1980). Composition of aphid alarm pheromones. Journal of Chemical Ecology, 6, 349–360.
Puente, M., Magori, K., & Kennedy, G. G. (2008). Impact of herbivore-induced plant volatiles on parasitoid foraging success: a spatial simulation of the Cotesia rubecula, Pieris rapae, and Brassica oleracea system. Journal of Chemical Ecology, 34, 595–970.
Roitberg, B. D., Myers, J. H., & Frazer, B. D. (1979). Influence of predators the movement of apterous pea aphids between plants. Journal of Animal Ecology, 48, 111–122.
Schmidt, M. H., Lauer, A., Purtauf, T., Thies, C., Schaefer, M., & Tscharntke, T. (2003). Relative importance of predators and parasitoids for cereal aphid control. Proceedings of the Royal Society London B, 270, 1905–1909.
Schwartzberg, E. G., Kunert, G., Rose, U. S. R., Gershenzon, J., & Weisser, W. W. (2008). Alarm pheromone emission by pea aphid, Acyrthosiphon pisum, clones under predation by lacewing larvae. Entomologia Experimentalis et Applicata, 128, 403–409.
Sisterson, M. S. (2008). Effects of insect-vector preference for healthy or infected plants on pathogen spread: insights for a model. Journal of Economic Entomology, 101, 1–8.
Sloggett, J. J., & Weisser, W. W. (2002). Parasitoids induce production of the dispersal morph of the pea aphid, Acyrthosiphon pisum. Oikos, 98, 323–333.
Sloggett, J. J., & Weisser, W. W. (2004). A general mechanism for predator- and parasitoid-induced dispersal in the pea aphid, Acyrthosiphon pisum. Aphids in a New Millennium, Science Update, pp 79–85.
Smyrnioudis, I. N., Harrington, R., Clark, S. J., & Katis, N. (2001). The effect of natural enemies on the spread of barley yellow dwarf virus (BYDV) by Rhopalosiphum padi (Hemiptera: Aphididae). Bulletin of Entomological Research, 91, 301–306.
Srinivasan, R. A., Alvarez, M. M., Eigenbrode, S. D., & Bosque-Perez, N. A. (2006). Influence of hairy nightshade Solanum sarrachoides (Sendtner) and Potato leafroll virus (Luteoviridae: Polerovirus) on the host preference of Myzus persicae (Sulzer) (Homoptera: Aphididae). Environmental Entomology, 35, 546–553.
Stearns, S. C. (1989). The evolutionary significance of phenotypic plasticity. BioScience, 39, 436–445.
Stout, M. J., Thaler, J. S., & Thomma, B. P. H. J. (2006). Plant-mediated interactions between pathogenic microorganisms and herbivorous arthropods. Annual Reviews, 51, 663–689.
Takabayashi, J., Dicke, M., & Posthumus, M. A. (1991). Variation in composition of predator-attracting allelochemicals emitted by herbivore-infested plants: relative influence of plant and herbivore. Chemoecology, 2, 1–6.
Turlings, T. C. J., Tumlinson, J. H., & Lewis, W. J. (1990). Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science, 250, 1251–1253.
Turlings, T. C. J., Tumlinson, J. H., Heath, R. R., Proveaux, A. T., & Doolittle, R. E. (1991). Isolation and identification of allelochemicals that attract the larval parasitoid, Cotesia marginiventris (Cresson), to the microhabitat of one of its hosts. Journal of Chemical Ecology, 17, 2235–2251.
Turlings, T. C. J., Alborn, H. T., Jones, T. H., Stenhagen, G., Loughrin, J. H., & Tumlinson, J. H. (1997). An elicitor of plant volatiles from beet armyworm oral secretion. Science, 276, 945–949.
van der Meijden, E., & Klinkhamer, P. G. L. (2000). Conflicting interests of plants and the natural enemies of herbivores. Oikos, 89, 202–208.
van Loon, J. J. A., der Boer, J. G., & Dicke, M. (2000). Parasitoid plant mutualism: parasitoid attack of herbivore increase plant reproduction. Entomologia Experimentalis et Applicata, 97, 219–227.
van Poecke, R. M. P., Posthumus, M. A., & Dicke, M. (2001). Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: chemical, behavioural, and gene-expression analysis. Journal of Chemical Ecology, 27, 1911–1928.
Verheggen, F. J., Arnaud, L., Bartram, S., Gohy, M., & Haubruge, E. (2008). Aphid and plant secondary metabolites induce oviposition in an aphidophagous hoverfly. Journal of Chemical Ecology, 34, 301–307.
Verheggen, F. J., Mescher, M. C., Haubrugge, E., De Moraes, C. M., & Schwartzberg, E. G. (2008). Emission of alarm pheromone in aphids: a non-contagious phenomenon. Journal of Chemical Ecology, 34, 1146–1148.
Vet, L. E. M., & Dicke, M. (1992). Ecology of infochemical use by natural enemies in a tritrophic context. Annual Review of Entomology, 37, 141–172.
Vet, L. E. M., Wackers, F. L., & Dicke, M. (1991). How to hunt for hiding hosts—the reliability-detectability problem in foraging parasitoids. Netherlands Journal of Zoology, 41, 202–213.
Wadhams, L. J., Birkett, M. A., Powell, W., & Woodcock, C. M. (1999). Aphids, predators and parasitoids. Novartis Foundation Symposium, 223, 60–67.
West-Eberhard, M. J. (1989). Phenotypic plasticity and the origins of diversity. Annual Review of Ecology and Systematics, 20, 249–278.
Witz, B. W., & Mushinsky, H. R. (1989). Pygidial secretions of Pasimachus subsulcatus (Coleoptera, carabidae) Deter predation by Eumeces inexpectatus (Squamata: Scincidae). Journal of Chemical Ecology, 15, 1033–1044.
Wohlers, P. (1980). Escape response of pea aphids, Acyrthosiphon pisum, to alarm pheromones and additional stimuli. Entomologia Expermentalis et Applicata, 27, 156–168.
Zehnder, G., Gurr, G. M., Kuhne, S., Wade, M. R., Wratten, S. D., & Wyss, E. (2007). Arthropod pest management in organic crops. Annual Review of Entomology, 52, 57–80.
Zhang, X. S., Holt, J., & Colvin, J. (2000). A general model of plant-virus disease infection incorporating vector aggregation. Plant Pathology, 49, 435–444.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeger, M., Chen, Z., Cunningham, E. et al. Population biology and epidemiology of plant virus epidemics: from tripartite to tritrophic interactions. Eur J Plant Pathol 133, 3–23 (2012). https://doi.org/10.1007/s10658-011-9913-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-011-9913-0