Abstract
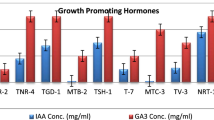
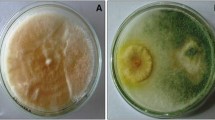
The annual crop loss caused due to phytopathogens is a serious problem affecting food security. To overcome the losses due to phytopathogens, the application of toxic pesticides is the only alternative but pose a serious threat to human and environmental health. Hence, more eco-friendly and sustainable approaches for efficient management of phytopathogens are urgently required. In this study, we evaluated the effectiveness of Trichoderma viride and Trichoderma harzianum as single as well as in consortium for elevating the systemic defense response and growth promotion activities in potato challenged with early blight pathogen Alternaria solani. The percent of mycelial inhibition of A. solani by T. viride (91.88%) and T. harzianum (80.11%) was recorded and compared with control. Scanning electron microscope (SEM) observations revealed the collapsed hyphae and sunken conidia of A. solani due to antagonistic activity of T. viride and T. harzianum. Induction of defense enzymes including total phenolic content (TPC), phenylalanine ammonia lyase (PAL), superoxide dismutase (SOD) and total protein content was increased 3.19, 3.72, 1.99 and 2.5 folds, respectively in consortia of Trichoderma spp. treated plants as compared to pathogen infected plants at 48 hapi. HPLC analysis demonstrated higher production free poly-phenolic compounds during combined application of Trichoderma spp. treated potato plants in the response of A. solani infection. This study demonstrates that the consortium of Trichoderma spp. is effective in elevating the host defense response by modulating the activities of phenylpropanoid derivatives, pathogenesis related proteins (PR-proteins), enzymes of oxidative stress network, and growth parameters in potato challenged with the early blight pathogen, A. solani












Similar content being viewed by others
Availability of data and material (data transparency)
The data would be made available on demand basis.
References
Abo-Elyousr, K. A. M., Hashem, M., & Ali, E. H. (2009). Integrated control of cotton root rot disease by mixing fungal biocontrol agents and resistance inducers. Crop Prot, 28, 295–301.
Ahn, I. P., Lee, S. W., & Suh, S. C. (2007). Rhizobacteria induced priming in Arabidopsis is dependent on ethylene, jasmonic acid, and NPR1. Mol Plant Microbe Interact, 20, 759–768.
Al-Mughrabi, K. I. (2005). Efficacy of oxidate for control of early blight (Alternaria solani) in potato storages. Plant Pathol. J., 4, 1–4.
Amin, F., Razdan, V. K., Mohiddin, F. A., Bhat, K. A., & Sheikh, P. A. (2010). Effect of volatile metabolites of Trichoderma species against seven fungal plant pathogens in vitro. J Phytol., 2, 34–37.
Awan, Z. A., & Shoaib, A. (2019). Combating early blight infection by employing Bacillus subtilis in combination with plant fertilizers. Current Plant Biology, 20, 100125.
Bisen, K., Ray, S., & Singh, S. P. (2019). Consortium of compatible Trichoderma isolates mediated elicitation of immune response in Solanum melongena after challenge with Sclerotium rolfsii. Archives of Phytopathology and Plant Protection, 52(7-8), 733–756.
Brueske, C. H. (1980). Phenylalanine ammonia lyase activity in tomato roots infected and resistant to the root knot nematode Meloidogyne incognita. Physiol Plant Pathol., 16, 409–414.
Buragohain, A. M., Das, B. C., & Islam, M. (2000). In-vitro studies of Trichoderma species against Sclerotium rolfsii Sacc. J Agric Sci., 13, 99–100.
Conrath, U., Beckers, G. J., Flors, V., Garcia-Agustin, P., Jakab, G., Mauch, F., Newman, M. A., Pieterse, C. M. J., et al. (2006). Priming: getting ready for battle. Mol Plant Microbe Interact, 19, 1062–1071.
Conrath, U. (2011). Molecular Aspects of Defence Priming. Trends in Plant Science, 16, 524–531.
Contreras-Cornejo, H.A., Macías-Rodríguez, L.I., Alfaro Cuevas, R., López-Bucio, J. (2014) Trichoderma improves growth of Arabidopsis seedlings under salt stress through enhanced root development, osmolite production and Na+elimination through root exudates. Mol Plant Microbe Interact 27,503–514.
Edin, E., Liljeroth, E., & Andersson, B. (2019). Long term field sampling in Sweden reveals a shift in occurrence of cytochrome b genotype and amino acid substitution F129L in Alternaria solani, together with a high incidence of the G143A substitution in Alternaria alternata. Eur. J. Plant Pathol., 155, 1–15.
Enespa, D., & S.K. (2014). Effectiveness of some antagonistic fungi and botanicals against Fusarium solani and Fusarium oxysporum f. sp. lycopersici infecting brinjal and tomato plants. Asian J Plant Pathol., 8, 18–25.
Fairchild, K. L., Miles, T. D., & Wharton, P. S. (2013). Assessing fungicide resistance in populations of Alternaria in Idaho potato fields. Crop Prot, 49, 31–39.
Fridovich, I. (1974). Superoxide dismutases. Adv EnzymolRelat Areas Mol Biol., 41, 35–97.
Fokkema, N. J. (1978). Fungal antagonism in the phylosphere. Ann Appl Biol, 89, 115–117.
Gadzouska, S., Delaunnay, A., Spasenoski, M., Maury, S., Joseph, C., & Hagege, D. (2007). Jasmonic acid elicitation of Hypericum perforatum L. Cell suspensions and effects on the production of phenylpropanoids adnnaphtodiantrones. Plant Cell Tissue Organ Cult., 89, 1–13.
Gallou, A., Cranenbrouck, S., Declerck, S. (2009) Trichoderma harzianum elicits defence response genes in roots of potato plantlets challenged by Rhizoctonia solani. Eur J Plant Pathol. 124, 219–230.
Harman, G.E., Howell, C.R., Viterbo, A., Chet, I., Lorito, I.M. (2004) Trichodermaspecies-Opportunistic, avirulent plant symbionts. Nature Rev 2, 43–56.
Hernandez, J.A., Ferrer, M.A., Jimenez, A., Barcelo, A.R., Sevilla, F. (2001) Antioxidant systems and O2) /H2O2 production in the apoplast of pea leaves. Its relation with salt induced necrotic lesions in minor veins. Plant Physiol 127, 827–831.
Jain, A., Singh, S., Sarma, B. K., & Singh, H. B. (2012). Microbial consortium mediated reprogramming of defence network in pea to enhance tolerance against Sclerotinia sclerotiorum. J ApplMicrobio, l112, 537–550.
Jetiyanon, K. (2007). Defensive-related enzyme response in plants treated with a mixture of Bacillus strains (IN937a and IN937b) against different pathogens. Biol Control, 42, 178–185.
Karthikeyan, M., Radhika, K., Mathiyazhagan, S., Bhaskaran, R., Samiyappan, R., & Velazhahan, R. (2006). Induction of phenolics and defense-related enzymes in coconut (Cocos nucifera L.) roots treated with biocontrol agents. Braz J Plant Physiol., 18, 367–377.
Keswani, C., Bisen, K., Chitara, M. K., Sarma, B. K., & Singh, H. B. (2017). Exploring the role of secondary metabolites of Trichoderma in tripartite interaction with plant and pathogens. In J. S. Singh & G. Seneviratne (Eds.), Agro-environmental sustainability (1st ed., pp. 63–79). Springer.
Kumar, D., Yusuf, M., Singh, P., Sardar, M., & Sarin, N. (2014). Histochemical Detection of Superoxide and H2O2 Accumulation in Brassica juncea Seedlings. Bio-Protocol., 2014, 4.
Lopez-Llorca, L. V., & Duncan, G. H. (1988). A study of fungal endoparasitism of the cereal cyst nematode Heterodera avenue by scanning electron microscopy. Can J Microhiol, 34, 613–619.
Lowry, O. H., Nira, J., Rosenburgh, A., Lewis, F., & Rose, J. R. (1951). Protein measurement with the folin phenol reagent. J Biol Chem, 193, 265–275.
Manganiello, G., Sacco, A., Ercolano, M. R., Vinale, F., Lanzuise, S., Pascale, A., Napolitano, M., Lombardi, N., Lorito, M., & Woo, S. L. (2018). Modulation of tomato response to Rhizoctonia solani by Trichoderma harzianum and its secondary metabolite harzianic acid. Front. Microbiol., 9, 1966.
Mayee, C.D., Datar, V.V. (1986) Phytopathometry. Tech. Bull-1 Marathwada Agric. Univ. Parbhani PP:66
Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci., 7, 405–410.
Moghaddam, G. A., Rezayatmand, Z., Nasr-Esfahani, M., & Khozaei, M. (2019). Genetic defense analysis of tomatoes in response to early blight disease. Alternaria alternata, Plant PhysiolBiochem, 142, 500–509.
Mokhtar, H., & Aid, D. (2013). Contribution in isolation and identification of some pathogenic fungi from wheat seeds, and evaluation of antagonistic capability of Trichoderma harzianum against those isolated fungi in vitro. AgriBiol J North Am., 4, 145–154.
Murmu, S., Dey, S., & Chakraborty, A. (2015). Management of early blight of potato using bio control agents and plant extracts. J. Appl Nat Sci., 7, 860–864.
Ngadze, E., Icishahayo, D., Coutinho, T. A., & van der Waals, J. E. (2012). Role of Polyphenol Oxidase, Peroxidase, Phenylalanine Ammonia Lyase, Chlorogenic Acid, and Total Soluble Phenols in Resistance of Potatoes to Soft Rot. Plant Disease, 96, 186–192.
Nicholson, R. L., & Hammerschmidt, R. (1992). Phenolic compounds and their role in disease resistance. Annu Rev Phytopathol, 30, 369–389.
Odilbekov, F., Edin, E., Mostafanezhad, H., Coolman, H., Grenville-Briggs, L. J., & Liljeroth, E. (2019). Within-season changes in Alternaria solani populations in potato in response to fungicide application strategies. Eur. J. Plant Pathol., 155, 953–965.
Pan, S. Q., Ye, X. S., & Kuc, J. (1991). Association of b-1,3 glucanase activity and isoform pattern with systemic resistance to blue mold in tobacco induced by stem injection with Peronospora tabacina or leaf inoculation with tobacco mosaic virus. Physiol Mol Plant Pathol., 39(1), 25–39.
Prasad, B. N., & Kumar, M. R. (2011). Comparative efficacy of different isolates of Trichoderma spp. against Rhizoctonia solani, incitant of sheath blight of rice. Ind J Fund Appl Life Sci., 1, 107–111.
Ragazzi, E., & Veronese, G. (1973). Quantitative analysis of phenolic compounds after thin layer chromatographic separation. J Chromatogr., 77, 369–375.
Rahman, M. A., Razvy, M. A., & Alam, M. F. (2013). Antagonistic activities of Trichoderma strains against chili anthracnose pathogen. Int J Microbiol Mycol., 1, 7–22.
Salisbury, F. B., & Ross, C. W. (1986). Plant physiology. CBS Publishers Distributors.
Sansinenea, E., Almaraz, M., Ramírez, M. D., & Ortiz, A. (2016). Cellular damage of plant pathogenic fungi by antifungal compound produced by Bacillus spp. isolates. Chem Ecol, 32, 722–732.
Segarra, G., Casanova, E., Bellido, D., Odena, M. A., Oliveira, E., & Trillas, I. (2007). Proteome, salicylic acid, and jasmonic acid changes in cucumber plants inoculated with Trichoderma asperellum strain T34. Proteomics., 7(21), 3943–3952.
Shaikh, F. T., & Sahera, N. (2013). In vitro assessment of antagonistic activity of Trichoderma harzianum against pathogen fungi. Int J Appl Res., 3, 57–59.
Shuman, J. L., & Christ, B. J. (2005). Integrating a host-resistance factor into the fast system to forecast early blight of potato. Am. J. Potato Res., 82, 9–19.
Singh, A., Sarma, B. K., Upadhyay, R. S., & Singh, H. B. (2013). Compatible rhizosphere microbes mediated alleviation of biotic stress in chickpea through enhanced antioxidant and phenylpropanoid activities. Microbiological Research, 15, 168(1), 33–40.
Singh, B. N., Singh, B. R., Singh, R. L., Prakash, D., Singh, D. P., Sarma, B. K., Upadhyay, G., & Singh, H. B. (2009). Polyphenolics from various extracts/fractions of red onion (Allium cepa) peel with potent antioxidant and antimutagenic activities. Food and Chemical Toxicology, 47, 1161–1167.
Singh, B.N., Singh, A., Singh, S.P., Singh, H.B. (2011) Trichoderma harzianum mediated reprogramming of oxidative stress response in root apoplast of sunflower enhances defence against Rhizoctonia solani. Eur J Plant Pathol. 131, 121–134.
Smirnoff, N. (1993) Tansley Review No. 52. The role of active oxygen in the response of plants to water deficit and desiccation. Plant Phytol. 125.
Srivastava, R., Khalid, A., Singh, U. S., & Sharma, A. K. (2010). Evaluation of arbuscular mycorrhizal fungus and Trichoderma harzianum formulation against Alternaria solani for the management of early blight of potato. Biological control., 53, 24–31.
Tapwal, A., & Pandey, H. (2016). In vitro evaluation of Trichoderma species for virulence efficacy on Botryodiplodia palmarum. Curr Life Sci., 2, 86–91.
Tapwal, A., Thakur, G., Chandra, S., & Tyagi, A. (2015). In-vitro evaluation of Trichoderma species against seed borne pathogens. Int J Biol Chem Sci., 1, 2349–2724.
Thordal-Christensen, H., Zhang, Z., Wei, Y., Collinge, D.B.(1997) Subcellular localization of H2O2 in plants, H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J.,11, 1187–1194.
Van der Ent, S., Van Wees, S. C., & Pieterse, C. M. (2009). Jasmonate signaling in plant interactions with resistance inducing beneficial microbes. Phytochemistry, 70, 1581–1588.
Viterbo, A., Harel, M., Horwitz, B. A., Chet, I., & Mukherjee, P. K. (2005). Trichoderma mitogen activated protein kinase signaling is involved in induction of plant systemic resistance. Appl Environ Microbiol., 71, 6241–6246.
Yedidia, I., Benhamou, N., & Chet, I. (1999). Induction of defense responses in cucumber plants (Cucumis sativus L.) by the biocontrol agent Trichoderma harzianum. Appl Environ Microbiol., 65, 1061–1070.
Zhang, D., Yu, S., Yang, Y., Zhang, J., Zhao, D., Pan, Y., Fan, S., Yang, Z., & Zhu, J. (2020). Antifungal effects of volatiles produced by Bacillus subtilis against Alternaria solani in Potato. Frontiers Microbiol., 11, 1196.
Zodor, R. E., & Anderson, A. J. (1992). Influence of root colonization bacteria on the defense responses in bean. Plant Soil., 140, 99–107.
Zivkovic, S., Stojanovic, S., Ivanovic, Z., Gavrilovic, V., Popovic, T., & Balaz, J. (2010). Screening of antagonistic activity of microorganisms against Colletotrichum acutatum and Colletotrichum gloeosporioides. Arch Biol Sci, 62, 611–623.
Funding
SK, RC and LB are grateful to Banaras Hindu University, Varanasi, India for financial support. The research was financially supported by the Ministry of Science and Higher Education of the Russian Federation project on the development of the Young Scientist Laboratory (no. LabNOTs-21-01AB) to CK.
Author information
Authors and Affiliations
Contributions
SK, CK, RC and ES were involved in the idea generation. SK, LB, CK conducted the experiments and analysed the data. SK, CK, ES were involved in manuscript preparation and editing. RC supervised the work.
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
The authors declare that there is no conflict of interest/ competing interests whatsoever.
Code availability (software application or custom code)
Not applicable
Additional declarations for articles in life science journals that report the results of studies involving humans and/or animals
Not applicable
Ethics approval (include appropriate approvals or waivers)
Not applicable
Consent to participate (include appropriate statements)
All authors have significantly contributed to this manuscript and agreed to submit this work in the European Journal of Plant Pathology.
Consent for publication (include appropriate statements)
All authors have approved the final draft of the manuscript and provided their consent for publication of the same in the European Journal of Plant Pathology.
Rights and permissions
About this article
Cite this article
Kumar, S., Chandra, R., Behera, L. et al. Dual Trichoderma consortium mediated elevation of systemic defense response against early blight in potato. Eur J Plant Pathol 162, 681–696 (2022). https://doi.org/10.1007/s10658-021-02431-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-021-02431-4