Abstract
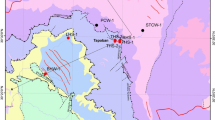
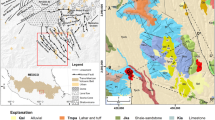
Chemical analyses of water samples from 19 hot and cold springs are used to characterize Takab geothermal field, west of Iran. The springs are divided into two main groups based on temperature, host rock, total dissolved solids (TDS), and major and minor elements. TDS, electrical conductivity (EC), Cl−, and SO4 2− concentrations of hot springs are all higher than in cold springs. Higher TDS in hot springs probably reflect longer circulation and residence time. The high Si, B, and Sr contents in thermal waters are probably the result of extended water–rock interaction and reflect flow paths and residence time. Binary, ternary, and Giggenbach diagrams were used to understand the deeper mixing conditions and locations of springs in the model system. It is believed that the springs are heated either by mixing of deep geothermal fluid with cold groundwater or low conductive heat flow. Mixing ratios are evaluated using Cl, Na, and B concentrations and a mass balance approach. Calculated quartz and chalcedony geothermometer give lower reservoir temperatures than cation geothermometers. The silica-enthalpy mixing model predicts a subsurface reservoir temperature between 62 and 90 °C. The δ18O and δD (δ2H) are used to trace and determine the origin and movement of water. Both hot and cold waters plot close to the local meteoric line, indicating local meteoric origin.













Similar content being viewed by others
References
Allison, A. W., Brown, D. S., Novo-Gradac, K. J. (1991). MINTEQA2/PRODEFA2, a geochemical assessment model for environmental systems: version 3.0 user’s manual. U.S. Environmental Protections Agency Report EPA/600/3-91/021.
Azizi, H., & Moinevaziri, H. (2009). Review of the tectonic setting of Cretaceous to Quaternary volcanism in northwestern Iran. Journal of Geodynamics, 47, 167–179.
Boni, M., Gilg, H. A., Balassone, G., Schneider, J., Allen, C. R., & Moore, F. (2007). Hypogene Zn carbonate ores in the Angouran deposit, NW Iran. Mineralium Deposita, 42, 799–820.
Cartwright, I., & Weaver, T. R. (2005). Hydrogeochemistry of the Goulburn Valley region of the Murray Basin, Australia: implications for flow paths and resource vulnerability. Hydrogeology Journal, 13, 752–770.
Clark, I. D., & Fritz, P. (1997). Environmental isotopes in hydrogeology. New York: Lewis Publishers.
Craig, H. (1961). Isotopic variations in meteoric waters. Science, 133, 1833–1834.
Daliran, F. (2003). Discovery of 1.2 kg/t gold and 1.9 kg/t silver in mud precipitates of a cold spring from the Takab geothermal field, NW Iran. In D. Eliopoulos et al. (Eds.), Mineral exploration and sustainable development (pp. 461–464). Rotterdam: Millpress.
Daliran, F. (2008). The carbonate rock-hosted epithermal gold deposit of Agdarreh, Takab geothermal field, NW Iran—hydrothermal alteration and mineralization. Mineralium Deposita, 43, 383–404.
Daliran, F., Pride, K., Walther, J., Berner, Z. A., & Bakker, R. J. (2013). The Angouran Zn (Pb) deposit, NW Iran: evidence for a two stage, hypogene zinc sulfide–zinc carbonate mineralization. Ore Geology Reviews, 53, 373–402.
Davraz, A. (2008). Hydrogeochemical and hydrogeological investigations of thermal waters in the Usak Area (Turkey). Environmental Geology, 54, 615–628.
Dotsika, E., Poutoukis, D., Michelot, J. L., & Kloppmann, W. (2006). Stable isotope and chloride, boron study for tracing sources of boron contamination in groundwater: boron contents in fresh and thermal water in different areas in Greece. Water, Air, and Soil Pollution, 174, 19–32.
Dotsika, E., Lykoudis, S., & Poutoukis, D. (2010). Spatial distribution of the isotopic composition of precipitation and spring water in Greece. Global and Planetary Change, 71, 141–149.
Favara, R. O., Grassa, F., Inguaggiato, S., & Valenza, M. (2001). Hydrogeochemistry and stable isotopes of thermal springs: earthquake-related chemical changes along Belice Fault (Western Sicily). Applied Geochemistry, 16, 1–17.
Fournier, R. O. (1981). Application of water geochemistry to geothermal exploration and reservoir engineering. In L. Rybach & L. J. P. Muffler (Eds.), Geothermal systems, principles and case histories. New York: Wiley.
Fournier, R. O., & Potter, R. W. (1979). Magnesium correction to the Na–K–Ca chemical geothermometer. Geochimica et Cosmochimica Acta, 43, 1543–1550.
Giggenbach, W. F. (1988). Geothermal solute equilibria. Derivation of Na–K–Mg–Ca geoindicators. Geochimica et Cosmochimica Acta, 52, 2749–2765.
Giggenbach, W. F., & Corrales, R. S. (1992). The isotopic and chemical composition of waters and steam discharges from volcanic magmatic-hydrothermal systems of the Guanacoste Geothermal Province, Costa Rica. Applied Geochemistry, 7, 309–332.
Giggenbach, W. F., & Glover, R. B. (1992). Tectonic regime and major processes governing the chemistry of water and gas discharges from the Rotorua geothermal field, New Zealand. Geothermics, 21, 121–140.
Giggenbach, W. F., Sheppard, D. S., Robinson, B. W., Stewart, M. K., & Lyon, G. L. (1994). Geochemical structure and position of the Waiotapu geothermal field, New Zealand. Geothermics, 23, 599–644.
GSI, Geological Survey of Iran. (1999). Geological map of Iran 1:100,000 series, Sheet 5463, Takht-e-Soleyman.
Gupta, S. K., Deshpande, R. D., Agarwal, M., & Raval, B. R. (2005). Origin of high fluoride in groundwater in the North Gujarat–Cambay region, India. Hydrogeology Journal, 13, 596–605.
Inguaggiato, S., Pecoraino, G., & D’Amore, F. (2000). Chemical and isotopical characterisation of fluid manifestations of Ischia Island (Italy). Journal of Volcanology and Geothermal Research, 99, 151–178.
Karimzadeh Somarin, A., & Lentz, D. R. (2008). Mineralogy, geochemistry and fluid evolution of a fossil hydrothermal system in the Paleogene Mendejin volcanic sequence, East Azarbaijan, Iran. Mineralogy and Petrology, 94, 123–143.
Kharaka, Y. K., & Mariner, R. H. (1989). Chemical geothermometers and their application to formation waters from sedimentary basins. In N. D. Naeser & T. H. McCulloh (Eds.), Thermal history of sedimentary basins (pp. 99–117). New York: Springer.
Kharaka, Y. K., Gunter, W. D., Affarwall, P. K., Perkins, E. H., De Braal, J. D. (1988). solmineq 88: a computer program code for geochemical modelling of water-rock interactions. In US Geological Survey Water Investigations Report (pp. 88–4227).
Larsen, D., Swihart, G. H., & Xiao, Y. (2001). Hydrochemistry and isotope composition of springs in the Tecopa basin, southeastern California, USA. Chemical Geology, 179, 17–35.
Modabberi, S., & Moore, F. (2004). Environmental geochemistry of Zarshuran Au-As deposit, NW Iran. Environmental Geology, 46, 796–807.
Möller, P., Rosenthal, E., Geyer, S., & Flexer, A. (2007). Chemical evolution of saline waters in the Jordan–Dead sea transform and in adjoining areas. International Journal of Earth Sciences, 96, 541–566.
Mutlu, H. (1998). Chemical geothermometry and fluid-mineral equilibria for the Omer-Gecek thermal waters, Afyon area, Turkey. Journal of Volcanology and Geothermal Research, 80, 303–321.
Naderi Siah Siahi, Gh. (2013). Hydrogheochemistry of heavy metals and geothermometry in Qurveh-Takab axis hydrothermal springs. M.sc. thesis Shiraz University, Shiraz. 159 pp.
Nicholson, K. (1993). Geothermal fluids, chemistry and exploration techniques. Berlin: Springer.
Parkhurst, D. L., & Appelo, C. A. J. (1999). User’s guide to PHREEQC (version 2)—a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. USGS Water-Resources Investigations Report pp. 99–4259.
Qin, D., Turner, J. V., & Pang, Z. (2005). Hydrogeochemistry and groundwater circulation in the Xi’an geothermal field, China. Geothermics, 34, 471–494.
Reed, M. H., & Spycher, W. H. (1984). Calculation of pH and mineral equilibria in hydrothermal waters with applications to geothermometry and studies of boiling and dilution. Geochimica et Cosmochimica Acta, 48, 1479–1492.
Saki, A. (2010). Proto-Tethyan remnants in northwest Iran: geochemistry of the gneisses and metapelitic rocks. Gondwana Research, 17, 704–714.
Sharifi, R., Moore, F., & Keshavarzi, B. (2014). Potential health risks of arsenic, antimony and mercury in the Takab geothermal field, NW Iran. International Journal of Environmental Studies, 71(3), 372–390.
Stockli, D., Hassanzadeh, J., Stockli, L., Axen, G., Walöker, J. D., & Terrence, T. J. (2004). Structural and geochronological evidence for Oligo-Miocene intra-arc low-angle detachment faulting in the Takab–Zanjan area, NW Iran. Geological Society of America Program, 36, 319.
Tarcan, G., & Gemici, Ű. (2003). Water geochemistry of the Seferihisar geothermal area, İzmir, Turkey. Journal of Volcanology and Geothermal Research, 126, 225–242.
Trusdell, A. H., & Fournier, R. O. (1977). Procedure for estimating the temperature of a hot-water component in a mixed water by using a plot of dissolved silica versus enthalpy. United States Geological Survey. Journal of Research, 5, 49–52.
Vengosh, A., Helvacı, C., & Karamanderesi, İ. H. (2002). Geochemical constraints for the origin of thermal waters from western Turkey. Applied Geochemistry, 17, 163–183.
Verma, M. P. (2001). Silica solubility geothermometers for hydrothermal systems. Water–rock interaction (pp. 349–352). Lisse: Swets and Zeitlinger.
Warner, N. R., Kresse, T. M., Hays, P. D., Down, A., Karr, J. D., Jackson, R. B., & Vengosh, A. (2013). Geochemical and isotopic variations in shallow groundwater in areas of the Fayetteville Shale development, north-central Arkansas. Applied Geochemistry, 35, 207–220.
Acknowledgments
The authors would like to acknowledge the help of Shiraz University Research Committee for financially supporting this research. Thanks are also extended to the Shiraz University Medical Geology Research Center and Center of Excellence for Geology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharifi, R., Moore, F., Mohammadi, Z. et al. Estimation of deepwater temperature and hydrogeochemistry of springs in the Takab geothermal field, West Azerbaijan, Iran. Environ Monit Assess 188, 75 (2016). https://doi.org/10.1007/s10661-015-5037-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-5037-x