Abstract
Since the middle of the 20th century, six species of Ponto-Caspian amphipods (Chaetogammarus ischnus, C. warpachowskyi, Chelicorophium curvispinum, Dikerogammarus haemobaphes, Obesogammarus crassus, Pontogammarus robustoides), one Baikalian amphipod Gmelinoides fasciatus and one amphipod of Atlantic origin Gammarus tigrinus have expanded in Russia and adjacent regions. A wide variety of human mediated vectors such as deliberate and accidental introductions, natural migration via constructed inland waterways and high rates of spread, survival and reproduction in these species have facilitated rapid dispersal and successful establishment of these alien species. Causes of successful establishment of these invaders and potential consequences of the invasions including extinctions of native species in rivers, lakes and estuaries of north-western Russia are discussed.
Similar content being viewed by others
Introduction
At present, the number of non-indigenous invertebrates in different parts of the world has increased, resulting in structural and functional changes of aquatic ecosystems (Cohen & Carlton, 1998; Leppäkoski et al., 2002). For example, during the last decade, five new amphipod species have been added to the list of fauna for Poland (Jażdżewski et al., 2002). The dispersal rate of a species and the ability to survive and reproduce in new conditions are important characteristics of invading species. As a rule, the dispersion of euryoecious species, including most amphipods, in different directions is a rapid process owing to the ability of a species to migrate great distances and successfully establish under new conditions. The aim of this paper is to analyse the possible pathways for recent amphipod invaders to European Russia, focusing mainly on the north-western region. Fundamental causes of invasion, or why a species was transported; possible invasion routes including known invasion corridors; vectors of invasions, or how a species is transported and consequences of their successful establishment are discussed.
Alien species of amphipods in inland waters of north-western Russia
There are eight amphipod species that have invaded north-western Russia and adjacent areas of the Baltic Sea basin (Table 1). Amphipods of the so-called Ponto-Caspian complex (Chaetogammarus ischnus (Stebbing), Chaetogammarus warpachowskyi (Sars), Chelicorophium curvispinum (Sars), Dikerogammarus haemobaphes (Eichwald), Obesogammarus crassus (Sars), Pontogammarus robustoides (Sars)) are the leading group in terms of number among these invaders. Three Ponto-Caspian species (C. curvispinum, O. crassus, P. robustoides) and one North-American Gammarus tigrinus Sexton have established in aquatic ecosystems of the Kaliningrad province in Russia (Ezhova et al., 2005). At least three species, C. warpachowskyi, P. robustoides and Baikalian Gmelinoides fasciatus (Stebbing) were recorded in the Gulf of Finland and aquatic ecosystems of the Leningrad province in Russia (Berezina & Panov, 2003a; Alimov et al., 2005). Findings of C. warpachowskyi were also reported from the Curonian Lagoon in Lithuania (Olenin & Leppäkoski, 1999; Jankauskiené, 2002). Recently, G. tigrinus was found along the Finnish coast in the Gulf of Finland (Pienimaki et al., 2004), from which it can penetrate the Russian area of the gulf. C. ischnus (Stebbing) was not found in the Russian part of the Baltic Sea, but presence of this species in the Vistula River (Poland) and in the Curonian Lagoon (Lithuania) was recorded (Jażdżewski, 1975; Jażdżewski & Konopacka, 2002; Jankauskiené, 2002). At present, two species D. haemobaphes and G. fasciatus are widely distributed in central Russia, including the Upper Volga basin. These species have the status of active migrants and may appear in other areas of Russia and adjacent countries in the next few years.
The uncontrolled spread of invasive amphipods, such as G. fasciatus and P. robustoides in aquatic ecosystems of Russia has had a high ecological impact, leading to changes in pre-existing biota, losses of species diversity and destabilization of recipient ecosystems through food web alterations. Other non-indigenous species belong to invaders with medium and low ecological impacts on the ecosystems, as they do not have the potential to dominate the ecosystems and often coexist with populations of native species without affecting the dynamic structure and stability of the ecosystem.
Causes for the increasing rate of amphipod invasions in Russia and the former USSR
Factors influencing the dispersal rate of alien amphipods, as well as, many other groups of aquatic and terrestrial organisms are often associated with human activities. In different cases the factors can act additionally or antagonistically with each other or with natural processes.
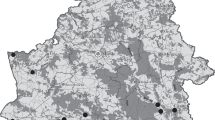
The destruction of natural barriers between different basins of Europe in the 19th and 20th centuries is considered to be one of the most important factors, which has resulted in range expansion of many species in different directions (Jażdżewski, 1980). The majority of alien amphipods penetrated the Baltic Sea basin from basins of the Volga River, Caspian Sea, Black Sea and the Sea of Azov after the construction of artificial canals, reservoirs and drainage systems and the formation of water routes, or so-called invasion corridors (Fig. 1). At present, three inland invasion corridors are known for alien species to extend through Europe from the Ponto-Caspian basin to the Baltic Sea (Bij de Vaate et al., 2002). The Volga-Don, Volga-Baltic, Dnieper-Vistula and Danube-Rhine waterways are the most important significance corridors in the dispersal of amphipods from the southern basin in a northern direction (Jażdżewski, 1980; Bij de Vaate et al., 2002; Slyn’ko et al., 2002). Construction of the Pripet-Bug canal (or King’s Canal) connecting the Dnieper and Vistula systems has resulted in the range expansion of several Ponto-Caspian amphipods (C. ischnus, C. curvispinum, P. robustoides) to the Baltic Sea and western Europe (Jażdżewski, 1980; Bij de Vaate et al., 2002; Jażdżewski et al 2004). At the beginning of the 20th century, the Oginski’ canal connecting the Dnieper and Neman systems facilitated the appearance of C. curvispinum in the Neman River and its dispersal to the Curonian Lagoon in the Baltic Sea, however, at present this old canal has no exit (Bij de Vaate et al., 2002). The Main-Danube canal, connecting the Rhine and Danube rivers, has also enabled four Ponto-Caspian species C. curvispinum, Obesogammarus obesus (Sars), Dikerogammarus villosus (Sowinsky), D. haemobaphes to enter the Rhine River from the Upper Danube River (Van der Velde et al., 2000; Bij de Vaate et al., 2002) and allowed one gammaridean amphipod of Balkan origin Gammarus roeselii Gervais to potentially spread into western Europe (Jażdżewski, 1980). Most likely, the Volga-Baltic waterway has played the main role in the dispersal of some amphipods (G. fasciatus, D. haemobaphes) as well as other crustaceans (Cornigerius maeticus, Cercopagis pengoi, Eriocheir sinensis, Evadne anonyx) in aquatic ecosystems of Russia. This waterway can also be considered as a possible invasion route of G. fasciatus from the Upper Volga basin to the Onega Lake (Berezina & Panov, 2003b).
Aquatic ecosystems of the north-western Russia and the Ponto-Caspian-Volga-Baltic waterway inhabited by alien amphipods: (1) Black Sea, (2) the Sea of Azov, (3) Caspian Sea, (4) Vistula Lagoon, (5) Curonian Lagoon, (6) Neva Estuary of the Gulf of Finland, (7) Lake Peipsi, (8) Lake Ladoga, (9) Lake Onega, (10) Lake Beloe, (11) Rybinsky Reservoir, (12) Gor’kovsky Reservoir, (13) Cheboksarsky Reservoir, (14) Kuibyshevsky Reservoir, (15) Volga River, (16) Don River, (17) Dnieper River
Climatic change, such as global warming, together with the formation of European inland waterways may also have facilitated the rapid dispersal of some aquatic species, including thermophilous amphipods from southern rivers of Ponto-Caspian and Mediterranean basins to aquatic systems of central and northern regions (Dukes & Mooney, 1999; Slyn’ko et al., 2002). Actually, during the 20th century the average temperature of the earth’s atmosphere was 1.0–3.5°C higher than in the 19th century (Houghton et al., 1996; Dukes & Mooney, 1999), which has resulted in the warmer winters at high latitudes.
Natural processes, as well as the downstream drift of benthic organisms in rivers could also favour the rapid spread of many amphipod species between different basins. For example, the total biomass of drifting crustaceans, including Corophium spp. in the Don River averaged 9 tons (wet weight) during the summer; although this weight increased significantly during the seasonal floods (Mordukhai-Boltovskoi, 1960). During flood periods in some locations of the Volga River, amphipods Obesogammarus sarsi (Sowinsky), D. haemobaphes, P. obesus and C. curvispinum have been transported downstream at a rate of >3,000 ind. per second (or 4.6 g s−1) when the width of the river is taken into account (Lyakhov, 1961).
The ability of most amphipods to migrate long distances is a common behavioural trait in freshwater, as well as, marine ecosystems and has facilitated their natural range expansion. There are vertical and horizontal migrations, with cyclic (diurnal, seasonal) or acyclic characteristics (Dedyu, 1980). It is known that G. fasciatus, the most active migrant in inland waters of Russia, was recorded as a dominant species among amphipods, migrating vertically from bottom to surface waters in Lake Baikal (Bessolitsyna, 2002). According to the migration theory by Birshtein (1935), the upstream migration of species in the rivers of the Sea of Azov and the Caspian and Black seas has resulted in the rapid dispersal of many amphipods from the south to the north of the former USSR. By the middle of the 20th century, the Ponto-Caspian amphipods C. ischnus, D. haemobaphes, D. villosus and O. obesus reached the middle part of the Volga River spreading upstream more than 4,000 km from their native area (Lyakhov, 1958; Mordukhai-Boltovskoi, 1960; Dedyu, 1980). In particular, the amphipod O. obesus extended its distribution area 500 km upstream in the river during a short period from 1958 to 1972 (Shakhmatova & Antonov, 1988). Amphipod migration also has an important significance, not only in species dispersal, but in feeding, reproduction, defence from predators and unfavourable factors, such as hypoxia, pollution, algae blooms and storms (Dedyu, 1967, 1980; Ioffe & Maximova, 1968).
In some cases, large specimens of amphipods, which can migrate for long distances were able to transport attached invertebrates (molluscs, rotifers and infusorians etc.) and can be considered as a possible vector of accidental introductions for other species of invertebrate. For example, it is known that specimens of D. villosus have transported juveniles of the mollusc Dreissena spp. during migration upstream in the Ponto-Caspian rivers (Dedyu, 1963).
Construction of artificial water-bodies, such as artificial ponds and reservoirs of power stations on the important rivers of Russia and related changes of habitat conditions, hydrodynamic stabilization, eutrophication and appearance of “empty” niches for new species have resulted in local dispersions of euryoecious species of amphipods outside their native areas. Thus, after construction of the Dnepropetrovsky Reservoir on the Dnieper River, amphipods P. robustoides and D. haemobaphes colonized the reservoir and became the dominant species in terms of density and biomass in the macrophyte beds, although they were very rare in the former part of the river (Mordukhai-Boltovskoi, 1960). In the Dubosarsky Reservoir, the amphipod density increased 2-fold in comparison with the density in the same part of the Dnieper River before reservoir construction. Species, such as C. curvispinum, C. ischnus and D. villosus came out of the Ponto-Caspian basin because of active reproduction in the constructed reservoirs and their subsequent upstream migration (Dedyu, 1967).
Disturbed habitats and unfavourable abiotic conditions of the former river during construction of reservoir resulted in a decrease in the abundance of native amphipods or a contraction in their range in this basin. For example, in the 1920s, the upper border of the distribution range of D. haemobaphes was situated in the Volga River near Yaroslavl city (Bening, 1924) however, by 1951–1972 it had dispersed downstream to near Nizhnyi Novgorod city (Shakhmatova & Antonov, 1988). The upstream migration of the amphipods in this case was limited by constructed dams partitioning the river bed and native populations were retained, so that a so-called “mosaic” distribution range of this species was established (Mordukhai-Boltovskoi, 1960; Zhadin, 1964).
At present, some Ponto-Caspian amphipods survive in the modern Volga River basin. For example, D. haemobaphes continues to migrate upstream from their known localities: in 1995–1997, it was recorded in the upper part of the Gor’kovsky Reservoir and in the Volga River near Yaroslavl city (Bakanov, 2003). Other findings of this species were reported from the Upper Moskva River in 1993 (L’vova et al., 1996), as well as in the Volga River near the site connected by a canal with the Moskva River in 1995 and finally, in the Rybinsky Reservoir in 1997 (A. Bakanov, pers. comm.). All these findings indicate an active range expansion by D. haemobaphes in a northern direction.
In some locations, intentional introductions of amphipods may have caused the extinction of native species. In the Gor’kovsky Reservoir, the Baikalian amphipod G. fasciatus was intentionally introduced for enrichment of fish production in the 1960s and has spread widely in to different biotopes over several years, replacing O. obesus and D. haemobaphes which were common species in the former part of the Volga River (Mordukhai-Boltovskoi & Chirkova, 1971; Mordukhai-Boltovskoi & Dziuban, 1976). In the 1970s, the distribution range of both species considerably contracted in the Volga River and has been limited near Kstovo city (Shakhmatova & Antonov, 1988). At the same time, introduced G. fasciatus dispersed from the Gor’kovsky Reservoir to other Volga Reservoirs (Cheboksarsky, Kuibyshevsky and others), successfully established itself and became the dominant species in the majority of the aquatic ecosystems of the Upper Volga basin (Borodich, 1979; Shcherbina et al., 1997). Similarly, in the Angara River (Siberia), before construction of its reservoirs, the number of amphipod species including G. fasciatus was high (up to 45 species). After construction of the Angara Reservoirs, species richness significantly decreased, the amphipods Eulimnogammarus verrucosus (Gerstfeldt) and E. viridis (Dybowsky), which were leading species in the former river disappeared, whereas G. fasciatus survived and rapidly occupied all types of habitat between depths of 0–5 m, becoming the dominant species in terms of density and biomass among the amphipods (Erbaeva et al., 2002).
During the 1950–1980s, large-scale intentional introductions of crustaceans were a major vector of amphipod invasions in inland waters of European Russia and Siberia. Enrichment of fish production was the principal motivation for the introductions. At least 30 amphipod species of Ponto-Caspian origin, 3 species of Siberian origin and 2 species belonging to so-called “glacial relicts” were used during amphipod transportations through the former USSR area (Gordeev, 1954; Gasiunas, 1972; Pirozhnikov & Ioffe, 1974; Karpevich, 1975; Jażdżewski, 1980; Zadoenko et al., 1985; Suschenya et al., 1986; Grigorovich et al., 2002). In addition, four species (Gammaracanthus lacustris Sars, Pallasea quadrispinosa Sars, G. tigrinus and G. pulex) were introduced to aquatic ecosystems of Western Europe (Jażdżewski, 1980; Fürst, 1981). These intentional introductions (or so-called acclimatizations) resulted in a rapid change of distribution area for many native amphipods.
The large-scale mass transportation of amphipods (even unknown species), conducted in the former USSR during the second part of the last century, were often not documented, which made it difficult to determine the invasion routes of acclimatized species. For example, it is still unclear how P. robustoides penetrated the eastern Gulf of Finland. This species (or mix of species) was taken from the Kaunas Reservoir for its intentional introduction into the aquatic ecosystems of the Leningrad Region in Russia (Gasiunas, 1972; Lazauskiene et al., 1995), however, the consequences of this introduction are unknown.
Accidental introductions of some alien species have taken place during contaminated acclimatizations of aquatic plants or animals. Introductions of aquatic plants have caused the invasion of G. roeselii from the southern Balkans to France (Jażdżewski, 1980; Jażdżewski & Roux, 1988). Baikalian G. fasciatus was accidentally introduced in Lake Peipsi during the intentional acclimatization of Gammarus lacustris Sars from the aquatic ecosystems of Siberia in the 1970s (Timm & Timm, 1993; Panov & Berezina, 2002).
Accidental introductions of species also occur with the ballast water of ships or in sediments of ballast tank, which are considered the main cause of species invasions throughout the world (Carlton, 1989; Ruiz et al., 2000). However, only a few case studies of this vector for amphipod invasions are known. For example, the North-American amphipods, G. tigrinus and Crangonyx pseudogracilis Bousfield were introduced in England with ballast water transport (Crawford, 1937; Hynes, 1955). The transoceanic transfer of the oligohaline amphipod C. ischnus from Europe to the Great Lakes of America occurred by the same vector (Witt et al., 1997; Cristescu et al., 2004). Despite the opening of inland waterways connecting the southern and northern regions of the European part of the former USSR and intensive shipping, introductions of amphipods are still unknown for Russia.
The survival of amphipods during transportation in ballast tanks (water or sediments) is possible in most cases because of the high tolerance of this group of animals to different abiotic factors, mainly salinity. All Ponto-Caspian amphipods are characterized by high euryhalinity, surviving in the range of 0.1–20‰ (Romanova, 1959; Mordukhai-Boltovskoi, 1960; Bruijs et al., 2001). The freshwater amphipod G. fasciatus, which is widely spread throughout Russia are able to survive at salinities less than 7–8‰ (Berezina et al., 2001; Berezina & Panov, 2003a). Another invader in the European waters, G. tigrinus, has been recorded along the coastal zone of North America, where water salinity changes from 1‰ to 25‰ (Bousfield, 1973).
Establishment of alien amphipods in aquatic ecosystems of Russia and the former USSR
Transfers of amphipods were frequently accompanied by physiological adaptations and changes in life cycle traits of introduced species in recipient ecosystems. Some authors (Mordukhai-Boltovskoi, 1960; Dedyu, 1967; Mordukhai-Boltovskoi & Chirkova, 1971) also recorded the significant morphological variability for C. warpachowskyi, G. fasciatus, P. robustoides, O. obesus, O. crassus and D. villosus in new conditions. This variability is a common feature for the majority of amphipods exposed to different conditions. A significant difference in body size, number of setae and structure of pereopods, uropods and telson were found in specimens of G. fasciatus within one region (Mekhanikova, 2000), which suggested a high molecular genetic polymorphism in this species.
Life cycle traits can change in response to the conditions of the recipient ecosystem. It is known that 1,000–1,250 day-degrees (or approximately 55–65 days at a temperature of 18.5°C) are needed for oogenesis of G. fasciatus. The number of generations, in the case of G. fasciatus, is temperature-dependent and ranges from 1 to 3 per year, with one generation in the littoral zone of Lake Baikal with day-degrees less than 1,200 during summer, two generations in north-eastern lakes with 1,500–2,000 day-degrees (Lake Ladoga, Lake Peipsi and Lakes of Karelian Istmus) and three generations in the Upper Volga reservoirs with the total day-degrees more than 2200 (Panov & Berezina, 2002).
In the recipient ecosystem, introduced species can occupy new types of habitats. For example, populations of D. haemobaphes, D. villosus and O. obesus are mainly concentrated in the deep-water habitats of southern rivers (Mordukhai-Boltovskoi, 1960; Dedyu, 1980), while in recipient reservoirs and estuaries of Russia they inhabit shallow areas with favourable trophic, oxygen and temperature conditions. At the same time, during storm or other unfavourable conditions, the amphipods are able to migrate into deeper water. In water-bodies of ancestral areas, the bulk of the G. fasciatus population is concentrated mainly into depths of 0.5–5.0 m, although it can inhabit depths up to 14 m, where its density is low (Bekman, 1962). The biomass of G. fasciatus had been recorded as extremely high (up to 5 kg m−2) during the summer in shallow zones of small water-bodies of the Baikalian basin. In contrast, in reservoirs and small rivers of the Upper Volga basin, this species was the most abundant in biocenosis of Dreissena spp. at depths of 6–10 m (Shcherbina et al., 1997).
The abrupt transfer of some species from one climatic geographic zone to another, without preliminary acclimation during intentional or accidental introductions, does not always result in successful establishment. Thus, introductions of Micruropus possolskii Sowinsky from the Angara River basin to reservoirs of Siberia and the Volga River (Ioffe, 1968; Volkov & Potina, 1977; Zadoenko et al., 1985) failed, as well as those of Gammarus lacustris from Siberia to Lake Peipsi (Timm & Timm, 1993; Panov et al., 2000) and P. robustoides from the Ponto-Caspian basin to the Simferopolsky and Kakhovsky reservoirs (Mordukhai-Boltovskoi, 1960). The transfer of some species within one region has also ended in the failure to establish. Such examples are known for Gammarus lacustris in some lakes of Ural and Siberia, including Lake Baikal (Deksbakh, 1952; Bekman, 1954; Safronov, 1993) and for the relict amphipods Gammaracanthus lacustris and Pallasea quadrispinosa in 40 % of Swedish lakes (Fürst, 1981; Hill et al., 1990). The cause of unsuccessful introductions is a discrepancy of hydrological, hydrochemical and trophic characteristics of recipient lakes and the ecological requirements of the introduced species. Nevertheless, the relict amphipods P. quadrispinosa and Gammaracanthus lacustris were successfully established in several lakes in Sweden (Hill et al., 1990) and Monoporeia affinis in Russia (Greze, 1958).
What life cycle traits are facilitating successful invasion of alien amphipods in new habitats? Alien species of amphipods, which belong to opportunistic or r-strategic species, will be able to increase their density over a short period, becoming leading species in recipient ecosystems. As a rule, high fecundity, fast growth and maturation of juveniles, wide food spectrum, high genetic variability and tolerance to different factors, including pollutants are the main characteristics of this alien amphipod species (Dennert, 1974; Dedyu, 1980; Van den Brink et al., 1993; Diсk et al., 1999; Holdich et al., 1999; Berezina & Panov, 2003a).
Possessing the majority of the above-mentioned characteristics, the Baikalian amphipod G. fasciatus could be considered as one of the most successful invaders in inland waters of Russia. Characteristic features of its life history facilitate successful adaptation and rapid population growth in recipient ecosystems. Reproduction of this species in the Neva Estuary and lakes of the Baltic Sea basin begins in March–April at a water temperature of 4–5°С. At 14–15°С, copulation of the amphipods lasts for 2 days; in 11–15 days fecund females produce fertile eggs in the brood pouch, and then after a further 15–18 days the females release juveniles (Nilova, 1976). Under favourable conditions, each mature female produces a maximum of 8–10 broods per season. Maximum clutch size in G. fasciatus is 34–36 eggs (sometimes up to 46 eggs) (Bekman, 1962; Vershinin, 1967; Savateeva, 1985; Skalskaya, 1996; Berezina, 2005).
During the reproductive period, the population structure of G. fasciatus is characterized by females prevailing over males, which can facilitate rapid population growth. This phenomenon is a common adaptation for many amphipod species and has been described for G. fasciatus populations in the Angara River reservoirs (Vershinin, 1967), Rybinsky Reservoir (Skalskaya, 1996), Lake Onega (Berezina & Panov, 2003b) and Neva Bay (Berezina, 2005). High summer temperatures can result in the rapid maturation of amphipods, with females producing their first clutch when their body size is only slightly larger than the juveniles. For example, 3.4 mm females of G. fasciatus were found with fecund eggs in July 2001 in the Neva Estuary at a water temperature of 27°С. In addition, in Lake Baikal (Posolskyi Bay), ovigerous females with a body length of 3.5 mm were recorded in late August (Bekman, 1962).
Gmelinoides fasciatus is able to survive at a wide range of temperatures, oxygen concentrations and ionic water content. This species, of freshwater origin, is widely spread in different aquatic ecosystems from oligotrophic Lake Onega, with a very low salt content to the oligohaline Neva Estuary (Baltic Sea). It inhabits silt, sandy, stony and woody substrates and concentrates in macrophyte and macroalgae beds (Phragmites, Eleocharis, Scirpus, Potamogeton, Cladophora and Nitella) (Panov & Berezina, 2002).
The important trait of alien species is the ability to adapt to conditions where food resources are limited by mixing feeding strategies. Omnivorous G. fasciatus consumes detritus, algae and aquatic plants. In addition, it preys upon small benthic and zooplankton organisms (Berezina et al., 2005). Gmelinoides fasciatus also tolerates moderate pollution and severe eutrophication. It was found to be among the first invertebrates to re-colonize previously lifeless locations caused by pulpmill discharges in Lake Ladoga (Panov, 1996). Similarly, other invasive species G. tigrinus and C. curvispinum were also found in ecosystems disturbed by anthropogenic pollution (Van den Brink et al., 1993; Lee & Bell, 1999). Gmelinoides fasciatus tolerates concentrations of oil products 5-times higher than the amphipod Нyalella azteca, and 20-times higher than the chironomid Chironomus riparius (Tomilina, 2000). Gmelinoides fasciatus was more tolerant to toxic concentrations of potassium than the molluscs Bithynia tentaculata, Planorbis planorbis, Sphaerium sp., Dreissena sp. and the isopod Asellus aquaticus. The tolerance range of G. fasciatus for potassium in water is 0.3–200 mg l−1 and LC50 for 240 h is 400–500 mg l−1. According to Makrushin (1998), the LC50 of the mollusc D. polymorpha for 240 h is 57 mg l−1 and the LC50 for the molluscs Unio sp. and Anodonta sp. is 170 mg l−1.
The main natural barriers for the establishment of G. fasciatus in new habitats are low pH (<6.0) (Nilova, 1976; Berezina, 2001) and salinity higher than 2‰ (Berezina et al., 2001), which prevents the successful completion of oogenesis. Soft water with a calcium content less than 5–7 mg l−1 and low pH have also terminated normal moulting in specimens (Bekman, 1962; Berezina, 2003). In addition, hypoxia in water and sediments is an unfavourable factor for the development of G. fasciatus (Bekman & Bazikalova, 1951).
The high resistance of other amphipod species to different unfavourable factors allows them to survive in many cases even at a lethal doze. For example, at oxygen concentrations of 0.1–0.3 mg l−1 for 1 h, specimens of D. villosus and C. ischnus became motionless, but after transfer to water with normal conditions they revived within 1 h (Mordukhai-Boltovskoi, 1960). It was revealed that P. robustoides was not able to reproduce successfully in water with potassium concentrations less than 10–15 mg l−1 (Berezina & Panov, 2003a), however, adult specimens were able to survive in distilled water for 1 week.
Introductions of a successful invader into new habitats, where limits for its range expansion such as predation, parasites and food competition are insignificant, result in rapid population growth of the invader. When the invader is introduced to such conditions, the classic ecological scenario begins (see Odum, 1975), where density (or biomass) initially increases slowly and imperceptibly, indicating the phase of “positive acceleration”. The following phase of population growth, the so-called “logarithmical phase”, is characterized by accelerated population growth rate and may result in a density explosion. As a rule, different environmental factors limit the logarithmical population growth, which results in the beginning of growth rate deceleration. The deceleration rate can be attributed to the phase of density stabilization, when the population size reaches a maximum level and may fluctuate slightly near this level. Population dynamics of alien amphipods in new habitats are often characterized by the above-mentioned scenario and this is demonstrated by the example of density growth in the Neva Estuary (Fig. 2). At the same time, the abrupt decline in the density of the first invader can be induced by new species invasions, especially in the case of species inhabiting the same niche (Fig. 3). Later, the density of both species can be stabilized near a certain level, which allows their coexistence.
Consequences of amphipod invasions in different aquatic ecosystems
The number of alien amphipods in Europe has considerably increased in recent years, however, only a few species can be considered as invasive or hazardous. According to the rule formulated by Williamson (Williamson & Fitter, 1996), only 10% (and 5–20% in the case of aquatic species) of established species can increase their density up to a significantly high level that they have an impact on the recipient ecosystem by facilitating a decrease its biodiversity and stability. However, we do not have universal criteria for the assessment of the minimum damage of an alien species, which defines it as harmful. One thing is clear; even a small number of specimens, possessing low genetic variability and low population density, may be sufficient for a generation to cause severe damage in a recipient ecosystem (Mack et al., 2000). According to Elton (1958), each introduction of a new species, whether an explosion of population density takes place or not, influences native populations and results in ecosystem destabilization.
Invasive species can interact with native species by predation, resource competition, habitat modification, causing the disappearance of species (or races) and irreversible losses of genetic diversity. The loss, or addition, of functionally dominant species, such as keystone species, ecosystem engineers or species with many trophic relations, can have a strong impact, inducing rapid losses of local biodiversity. Invasiveness of the alien species is mainly related to species characteristics, hierarchical complexity of the recipient ecosystem and its vulnerability. The vulnerability of the ecosystem increases depending on the degree of anthropogenic disturbance (Elton, 1958; Simberloff, 1981; Kinzelbach, 1995; Lozon & MacIsaac, 1997).
Although patterns of amphipod invasions in the Baltic Sea basin and Europe are well-known, processes of alien species interactions with native species and impacts on the environment are poorly understood. It is known that the impact of established invaders may be positive, neutral or negative and dependent on characteristics of the invader (Holdich et al., 1999; Westman, 2002). As for amphipods, these characteristics may include a larger body, faster growth, more aggressive behaviour, greater fecundity or better tolerance to unfavourable abiotic factors and pollution than in native species (discussed by Mordukhai-Boltovskoi, 1960; Dennert, 1974; Dedyu, 1980; Dick et al., 1999; Van der Velde et al., 1999).
After establishment of an alien species, its population size, feeding habits and its position in the food web may be significant in determining the type and strength of effect on the invaded community (Nyström et al., 1999). Most amphipod species are omnivorous, and the food preferences of each species may differ and be dependent on food resources in the invaded habitat. Adult specimens of different amphipod species are able to predate. Some species of the family Corophiidae, including C. curvispinum are suspension-feeders. Large-sized species D. villosus and Pontogammarus robustoides can be considered as the most effective predators (Berezina & Panov, 2003a; Krisp & Maier, 2005). Pontogammarus robustoides start to predate at a young age (body length of 6–7 mm), attacking the larvae of chironomids and oligochaetes, which are often larger than itself. In experiments, it was shown that specimens of D. villosus actively attacked Asellus sp., Gammarus duebeni, larvae of mayflies, chironomids and even aquatic beetles (Dick et al., 2002). Isotope analysis (δ15N) revealed that D. villosus has the same trophic status as the benthivorous fish level (Marguillier et al., 1998).
Predation of alien amphipods on small benthic organisms is often considered to be the main negative effect observed in a recipient community. In the stony littoral zone of the Neva Bay, predation of P. robustoides on benthic organisms (isopods, oligochaetes, aquatic insects) resulted in a significant decrease in their density (Berezina & Panov, 2003a). The intraguild predation was the primary mechanism, whereby the abundance of native amphipods (or earlier invaders) dropped abruptly after the establishment of a new amphipod species. This mechanism was found experimentally, in the case of the replacement of G. duebeni by G. pulex (Dick & Platvoet, 1996; Dick et al., 1999) as well as in two other cases of replacement, including G. fasciatus by P. robustoides (Berezina & Panov, 2003a) and G. lacustris by G. fasciatus (Berezina, pers. obs.). The phenomenon of the replacement of one amphipod species by another is a common trait for this group. Species interactions among amphipods of different origins have been observed in many cases (Martynov, 1932; Mordukchai-Boltovskoi, 1960; Dedyu, 1980). Antagonistic relationships have been distinguished between the species pairs of G. fasciatus and G. pulex, G. duebeni and G. pulex (Hynes, 1954; Dick et al., 1994), G. duebeni and G. salinus (Kinne, 1954), D. villosus and G. duebeni, D. villosus and G. tigrinus (Dick et al., 2002), G. lacustris and G. fasciatus, G. fasciatus and P. robustoides (Berezina & Panov, 2003a).
As a rule, the species which occurred in conditions within its tolerance range was more successful during species interactions. This scenario is illustrated by the example of the oligohaline amphipod G. tigrinus and the freshwater G. pulex interaction, when the displacement of the first species by the second was observed in waters with low ionic content, and the second by first ones in oligohaline estuaries (Van der Velde et al., 2000). In different aquatic ecosystems in Russia, a considerable decline in density or even the disappearance of the native G. lacustris was recorded in the case of G. lacustris and G. fasciatus coexistence (Panov, 1996; Panov et al., 2000; Berezina, 2005). Whilst G. fasciatus has a short life cycle and high fecundity that allows it to reach high densities in a short-term period, G. lacustris is characterized by low density, a longer life cycle, low reproductive potential and, in most cases, low density. Also, G. lacustris is less tolerant to storm activity (Bekman, 1954), which may limit its spread to the exposed littoral zone of large lakes and estuaries.
High densities of alien amphipods can facilitate the transformation and mineralization of organic matter and energy mediation in the coastal zone of the Baltic Sea estuaries (Berezina & Panov, 2003a). The intensity of these processes can be related to the consumption rates, depending on water temperatures, type of food items, age and consumer weight (Greze, 1977; Sushchenya, 1975). At 20°С, the rates of G. fasciatus and P. robustoides comprised 20–130% of individual consumer weight per day (Berezina & Panov, 2003a). Fecal pellets of amphipods consist of incompletely digested remains of animal and plant origin, and associated bacteria groups, which can be used by detritivorous invertebrates (oligochaetes, insect larvae and juveniles of amphipods and isopods). In addition, grazing by the amphipods G. fasciatus and P. robustoides can control the biomass of filamentous algae that is a cause of pollution in the coastal zone of the Gulf of Finland (Berezina et al., 2005). Similarly, Gasiunas (1975) revealed that the established population of P. robustoides with densities up to 4,980 ind. m−2, feeding on Cladophora algae, contributed to the disappearance of the algae in some lakes of Lithuania 5 years after introduction.
The successful invasion of alien amphipods in communities with low species diversity, have often resulted in enrichment of food resources for fish. It is known that the amphipod Gmelinoides fasciatus is a favourite food items for many fish species, such as perch, roach, bream, dace and whitefish (Leuciscus leuciscus). According to Mitskevich (1981), G. fasciatus played a significant role in feeding of benthophagous fish from Lake Otradny (Baltic Sea basin). Gmelinoides fasciatus juveniles were also a regular component, comprising 8.2–10% of the diet of bream and roach. In addition, these amphipods constituted about 65% of the biomass ingested by perch Perca fluviatilis.
Alien species inhabiting new ecosystems can transfer new species of parasites to vertebrates, as well as fish, birds and mammals. Amphipods are intermediate hosts for certain species of Acanthocephala, trematodes and microsporidia (Baldanova & Pronin, 2001). The Baikalian amphipod G. fasciatus is able to transfer the parasite Polymorphus magnus which can cause disease in ducks (Sidorov, 1963).
Conclusion
The major factors accelerating the dispersal process of many amphipods in European Russia are related to human activity. The destruction of natural barriers between the different basins of Europe is considered to be one of the most important factors, which has resulted in the range expansion of many amphipod species in different directions. Disturbed habitats are more often invaded by new species than well-balanced, undisturbed ones. The destruction of natural habitats will increase ecosystem invasiveness and facilitate the successful invasion of undesirable euryoecious species. The success of invading species strongly depends on life cycle traits, including reproduction rate, tolerance to environmental factors and the strength of their interactions with other species. In the recipient ecosystem, the established alien species can become the dominant species which can strongly influence the ecosystem. The consequences of amphipod introductions need further assessment, because it is clear that the impact of alien species on invading ecosystem can result in the serious loss of biodiversity and the distruption of system stability.
References
Alimov, A. F., M. I. Orlova, A. E. Antsulevich, T. F. Florinskaya, N. A. Berezina, I. V. Telesh, A. A. Maksimov & L. F. Litvinchuk, 2005. Biological pollution of aquatic ecosystems of the Gulf of Finland basin. In Golubev D. A. & N. D. Sorokin (eds), Preservation of the Environment, Rational Use and Safeguarding of Ecological Security in Saint Petersburg in 2004 (Okhrana okruzhayushchei sredy, prirodopol’zovanie i obespechenieecologicheskoi bezopasnosti v Sankt-Peterburge v 2004 godu). Sezam, Sankt-Peterburg: 185–194.
Arbaciauskas, K., 2002. Ponto-Caspian amphipods and mysids in the inland waters of Lithuania: history of introduction, current distribution and relations with native malacostracans. In Leppäkoski E., S. Olenin & S. Gollasch (eds), Invasive Aquatic Species of Europe. Distribution, Impacts and Management. Kluwer Academic Publishers, Dordrecht, Boston, London: 104–115.
Bakanov, A. I., 2003 Current status of benthos of the Upper Volga River within Yaroslavl region. Biologiya vnutrennikh vod 1: 81–88 (in Russian).
Baldanova, D. P. & N. M. Pronin, 2001. Gammarids as intermediate hosts of acanthocephalan parasites (Acanthocephala). In Takchteev, V. V. (ed), Investigations of Fauna of Aquatic Ecosystems of Eastern Siberia. Proceedings of Irkutsk State university (Issledovaniya fauny vodoemov Vostochnoi Sibiri. Sbornik trudov Irkutskogo Gosudarstvennogo universiteta). Izdatel’stvo IrGU, Irkutsk: 50–54 (in Russian).
Bekman, M. Y., 1954. Biology of Gammarus lacustris Sars from Lake Baikal basin. Proceedings of Baikalian Limnological Station (Trudy Baikal’skoi Limnologicheskoi stantsii) 14: 263–311 (in Russian).
Bekman, M. Y., 1962. Ecology and production of Micruropus possolskyi Sow. and Gmelinoides fasciatus Stebb. Proceedings of Limnological institute of Siberian department of Academy of sciences of USSR (Trudy Limnologicheskogo instituta Sibirskogo otdeleniya Akademii nauk SSSR) 22: 141–155 (in Russian).
Bekman, M. Y. & A. Y. Bazikalova, 1951. Biology and production capability of some Baikalian and Siberian amphipods. Proceedings of thematic meeting of Zoological Institute of Academy of Sciences of USSR (Trudy problemnogo i tematicheskogo soveshchaniya Zoologicheskogo instituta Akademii nauk SSSR) 1: 61–67 (in Russian).
Bening, A. L., 1924. To study of bottom life in the Volga River. Monographs of Volga Biological station 1. Saratov (in Russian).
Berezina, N. A., 2001. Influence of ambient pH on freshwater invertebrates under experimental conditions. Russian Journal of Ecology 5: 343–351.
Berezina, N. A., 2003. Tolerance of freshwater invertebrates to changing salinity of water. Russian Journal of Ecology 4: 261–266.
Berezina, N. A., 2005. Seasonal dynamics and fecundity of Gmelinoides fasciatus (Stebbing 1899) (Amphipoda: Crustacea) in macrophyte zone of northern Neva Bay (Gulf of Finland, Baltic Sea). Zoologichesky Zhurnal 4: 411–419 (in Russian).
Berezina, N. A., S. M. Golubkov & J. I. Gubelit, 2005. Grazing effects of alien amphipods on macroalgae in the littoral zone of the Neva Estuary (eastern Gulf of Finland, Baltic Sea). Oceanological and Hydrobiological Studies 34(1): 63–82.
Berezina, N. A., V. V. Khlebovich, V. E. Panov & N. V. Zaporozhets, 2001. Salinity resistance in the amphipod Gmelinoides fasciatus (Stebb.), introduced into the Gulf of Finland (Baltic Sea) basin. Doklady Academy Nauk 379(3): 414–416.
Berezina, N. A. & V. E. Panov, 2003a. Establishment of new gammarid species in the eastern Gulf of Finland (Baltic Sea) and their effects on littoral communities. Proceedings of Estonian Academy of Sciences. Biology. Ecology 52(3): 284–304.
Berezina, N. A. & V. E. Panov, 2003b. Establishment of the Baikalian amphipod Gmelinoides fasciatus (Amphipoda, Crustacea) in Lake Onega. Entomological review 83: S171–S174.
Berezina, N. A. & V. E. Panov, 2004. Distribution, population structure and salinity resistance of the invasive amphipod Gmelinoides fasciatus (Stebbing) in the Neva Estuary (Gulf of Finland, Baltic Sea). Hydrobiologia 514: 199–206.
Bessolitsyna, I. A., 2002. Night vertical migration of benthic amphipods in Lake Baikal. Isdatel’stvo Instituta geografii Sibirskogo otdeleniya Rossiiskoi Academii Nauk, Irkutsk (in Russian).
Birshtein, Y. A., 1935. About origin of marine crustaceans in rivers of Ponto-Caspian basin. Zoologichesky Zhurnal 14(4): 749–761 (in Russian).
Bij de Vaate, A., K. Jażdżewski, H. A. M. Ketelaars, S. Gollasch & G. Van der Velde, 2002. Geographical patterns in range extension of Ponto-Caspian macroinvertebrate species in Europe. Canadian Journal of Fisheries and Aquatic Sciences 59: 1159–1174.
Borodich, N. D., 1979. Baikalian amphipod Gmelinoides fasciatus (Stebbing) (Amphipoda, Gammaridae) in Kuibyshevsky Reservoir. Zoologichesky Zhurnal 58: 920–921 (in Russian).
Bousfield, E. L., 1973. Shallow-water gammaridean Amphipoda of New England. Comstock Publishing Associates a division of Cornell University press, Ithaca & London.
Bruijs, M. C. M., B. Kelleher, G. van der Velde & A. bij de Vaate, 2001. Oxygen consumption, temperature and salinity tolerance of the invasive amphipod Dikerogammarus villosus: indicators of further dispersal via ballast water transport. Archiv für Hydrobiologie 152: 633–646.
Carlton, J. T., 1989. Man’s role in changing the face of the ocean: biological invasions and implications for conservation of nearshore environments. Conservation Biology 3: 265–273.
Cohen, A. N. & J. T. Carlton, 1998. Accelerating invasion rate in a highly invaded estuary. Science 279: 555–558.
Crawford, G. I., 1937. An amphipod, Eucrangonyx gracilis, S. I. Smith, new to Britain. Nature, London 139: 327.
Cristescu, M., J. D. S. Witt, I. A. Grigorovich, P. D. N. Hebert & H. J. MacIsaac, 2004. Dispersal of the Ponto-Caspian amphipod Echinogammarus ischnus: invasion moves from the Pleistocene to the present. Heredity 92: 197–203.
Dedyu, I. I., 1963. On role of amphipods in geographical dispersion of dreissenid mollusks. Reports of Academy of sciences of Moldavian SSR. (Izvestiya Academii nauk Moldavskoi SSR) 5: 4–65 (in Russian).
Dedyu, I. I., 1967. Amphipods and Mysids of the Dnestr River and Prut River Basin. Nauka, Moskva (in Russian).
Dedyu, I. I., 1980. Amphipods of Fresh and Brackish Waters of the South-West of USSR. Shtiintsa, Kishinev (in Russian).
Dennert, H. G., 1974. Tolerance differences and interspecific competition in three members of the amphipod genus Gammarus. Bijdragen tot de Dierkunde (Contributions to Zoology) 44(1): 83–99.
Deksbakh, N. K., 1952. Gammarus lacustris in water-bodies of the middle Ural River and adjacent areas (dispersal, ecology, management). Proceedings of All-Union Hydrobiological Society (Trudy vsesoyuznogo gidrobiologicheskogo obshchestva) 4: 187–199 (in Russian).
Dick, J. T. A., R. W. Elwood & W. I. Montgomery, 1994. Range expansion of the alien amphipod Gammarus pulex in the River Lagan. Irish Nature Journal 23: 403–404.
Dick, J. T. A., R. W. Montgomery & R. W. Elwood, 1999. Intraguild predation may explain an amphipod replacement: evidence from laboratory populations. Journal of Zoology, London 249: 463–468.
Dick, J. T. A. & D. Platvoet, 1996. Intraguild predation and species exclusions in amphipods: the interaction of behaviour, physiology and environment. Freshwater Biology 36: 375–383.
Dick, J. T. A., D. Platvoet & D. W. Kelly, 2002. Predatory impact of the freshwater invader Dikerogammarus villosus (Crustacea: Amphipoda). Canadian Journal of Fisheries and Aquatic Sciences 59: 1078–1084.
Dukes, J. S. & H. A. Mooney, 1999. Does global change increase the success of biological invaders? Trends in Ecology and Evolution 14: 135–139.
Elton, C. S., 1958. The Ecology of Invasions by Animals and Plants. Methuen, London.
Erbaeva, E. A., G. P. Safronov & T. I. Kishchuk, 2002. Fauna of bottom invertebrates of Bratsky Reservoir. Biologiya vnutrennikh vod 1: 15–22 (in Russian).
Ezhova, E., L. Żmudzinski & K. Maciejewska, 2005. Long-term trends in the macrozoobentos of the Vistula Lagoon, southeastern Baltic Sea: species composition and biomass distribution. The Bulletin of the Sea Fisheries Institute 164(1): 55–73.
Fürst, M., 1981. Results of introductions of new fish food organisms into Swedish lakes. Reports of Institute of Freshwater Research, Drottningholm 59: 33–47.
Gasiunas, I. I., 1972. Enrichment of folder basis of water bodies of Lithuania by acclimatized crustaceans from Caspian Sea complex. In Virbitskas J. (ed), On the Breeding of Fish and Crustaceans in the Water Bodies of Lithuania (Voprosy razvedeniya ryb i rakoobraznykh v vodoemakh Litvy), Vilnius: 57–68 (in Russian).
Gasiunas, I. I., 1975. Peracarida of Dusya Lake (Baltic Sea basin). Gidrobiologicheskyi Zhurnal 11(1): 46–50 (in Russian).
Gordeev, O. H., 1954. Introduction of crustaceans as a food for fish in lakes of Karelian-Finnish SSR. Proceedings of meeting on problem of fish production enrichment in inland lakes of Karelian-Finnish SSR (Materyaly soveshchaniya po probleme povysheniya ryboproductivnosti vnutrennikh vodoemov Karelo-Finskoi SSR, sozvannogo Karelo-Finskim Filialom AN SSSR i Karelo-Finskim otdeleniem BNIORKh, Petrazavodsk): 152–161 (in Russian).
Greze, V. N., 1958. Relict mysis (Mysis oculata v. relicta) and pontoporean amphipod (Pontoporeia affinis) as objects of acclimatization. Zoologicheskyi Zhurnal 37(10): 1449–1461 (in Russian).
Greze, I. I., 1977. Amphipods of Black Sea and their biology. Naukova dumka, Kiev (in Russian).
Grigorovich, I. A., H. J. MacIsaac, N. V. Shadrin & E. L. Mills, 2002. Patterns and mechanisms of aquatic invertebrate introductions in the Ponto-Caspian region. Canadian Journal of Fisheries and Aquatic Sciences 59: 1189–1208.
Hill, C., M. Fürst & J. Hammer, 1990. Introduction of the amphipods Pallasea quadrispinosa and Gammaracanthus lacustris into lakes in northern Sweden. Annales Zoologici Fennici 27: 241–244.
Holdich, D. M., W. D. Rogers & J. D. Reynolds, 1999. Native and alien crayfish in the British Isles. In Gherardi F., & D.M. Holdich (eds), Crayfish in Europe as Alien Species. How to Make the Best of a Bad Situation. Brookfield, Balkema, Rotterdam: 221–235.
Houghton, J. T. (ed), 1996. Climate Change 1995: The Science of Climate Change. Contribution of Working Group 01I to the second assessment report of the intergovernmental panel of Climate Change. Cambridge University Press.
Hynes, H. B. N., 1954. The ecology of Gammarus duebeni Lilljeborg and its occurrence in freshwater in western Britain. Journal of Animal Ecology 23: 38–84.
Hynes, H. B. N., 1955. Distribution of some freshwater Amphipoda in Britain. Proccedings of International Association of Theoretical and Applied Limnology (Verhandlungen International Veréinigung fur Theoretisch und Angewandt Limnologie) 12: 620–628.
Ioffe, T. I., 1968. Review about actions of acclimatization of invertebrates as fish food in reservoirs. Proceedings of State Research Institute on Lake and River Fisheries (Trudy Gosudarstvennogo nauchno-issledovatel’skogo instituta ozernogo i rechnogo khozyaistva) 71: 7–29 (in Russian).
Ioffe, T. I. & L. P. Maximova, 1968. Biology of some crustaceans used for acclimatization into reservoirs. Proceedings of State Research Institute on Lake and River Fisheries (Trudy Gosudarstvennogo nauchno-issledovatel’skogo instituta ozernogo i rechnogo khozyaistva) 67: 87–104 (in Russian).
Jankauskiené, R., 2002. The density-biomass seasonal dynamics and diel vertical migration of Ponto-Caspian higher crustaceans and fish larvae in the littoral zone of the Curonian Lagoon. Ecologija 4: 21–28 (in Lithuanian).
Jażdżewski, K., 1975. Morfologia, laksonomia I wystepovanie w Polsce kielzy z rodzajow Gammarus Fabr. and Chaetogammarus Mart. (Crustacea, Amphipoda). Acta Universitatis Lodziensis. 185 p (in Polish).
Jażdżewski, K., 1980. Range extensions of some gammaridean species in European inland waters caused by human activity. Crustaceana supplement 6: 84–106.
Jażdżewski, K. & A. Konopacka, 2002. Invasive Ponto-Caspian species n Waters of the Vistula and Oder basins and the southern Baltic Sea. In Leppäkoski E., S. Olenin, & S. Golasch (eds), Invasive Aquatic Species of Europe. Distribution, Impacts and Management. Kluwer Academic Publishers, Dordrecht, Boston, London: 384–398.
Jażdżewski, K., Konopacka, A. & Grabowski M., 2002. Four Ponto-Caspian and one American gammarid species (Crustacea: Amphipoda) recently invading Polish waters. Contribution to Zoology 71(4): 115–122.
Jażdżewski, K. & A. L Roux, 1988. Biogeographie de Gammarus roeselii Gervais en Europe, en particulier repartition en France et en Pologne. Crustaceana 13: 272–277.
Jażdżewski, K., Konopacka, A. & M. Grabowski, 2004. Recent drastic changes in the gammarid fauna (Crustacea, Amphipoda) of the Vistula River deltaic system in Poland caused by alien invaders. Diversity and distributions 10: 81–87.
Karaman, G. S. & S. Pinkster, 1977. Freshwater Gammarus species from Europe, North Africa and adjacent regions of Asia (Crustacea, Amphipoda). II. Gammarus roeselli- group and related species. Bijdragen tot de Dierkunde (Contribution to zoology) 47(2): 165–196..
Karpevich, A. F., 1975. Theory and Practice of Acclimatization of Aquatic Organisms. Nauka, Moskva (in Russian).
Kinne, O., 1954. Interspezifische Sterilpaarung als konkurrenz-oecologischer Factor bei Gammariden (Crustacea, Peracarida). Naturwissenschaften. 18: 434–435.
Kinzelbach, R., 1995. Neozoans in European waters. Exemplifying the worldwide process of invasion and species mixing. Experientia 51: 526–538.
Krisp, H. & G. Maier, 2005. Consumption of macroinvertebrates by invasive and native gammarids: a comparison. Journal of Limnology 64(1), 55–59.
Lazauskiene, L. A., G. I. Vainotis & A.Y. Razinkov, 1995. Results of acclimatization of invertebrates in Baltic region. In Kudersky L. A. (eds), Results of Acclimatization of Aquatic Organisms (Rezul’taty rabot po akklimatizatsii vodnykh organizmov). Nauka, Sankt-Peterburg: 167–178 (in Russian).
Lee, C. E. & M. A. Bell, 1999. Causes and consequences of recent freshwater invasions by saltwater animals. Trends in Ecology & Evolution (TREE) 14(7): 284–288.
Leppäkoski, E., S. Olenin & S. Gollasch, (eds), 2002. Invasive Aquatic Species of Europe. Distribution, Impacts and Management. Kluwer Academic Publishers, Dordrecht, Boston, London.
Lozon, J. D. & H. J. MacIsaac, 1997. Biological invasions: are they dependent on disturbance. Environmental Review 5: 131–144.
Lyakhov, S. M., 1958. On distribution areas of Caspian amphipods in Volga river to the beginning of its technical reconstruction. Research reports of High School. Biological sciences (Nauchnye doklady Vysshei shkoly. Biologicheskie nauki) 3: 16–19 (in Russian).
Lyakhov, S. M., 1961. Floating benthos in Volga River near Kuibyshev before its regulation. Proceedings of All-Union Hydrobiological Society (Trudy Vsesoyuznogo Gidrobiologicheskogo obshchestva) 11: 150–161 (in Russian).
L’vova, A. A., A. V. Paliy & N. Y. Sokolova, 1996. Ponto-Caspian invaders in the Moskva River and in Moskva city. Zoologicheskiy Zhurnal 75(8): 1273–1274 (in Russian).
Makrushin, A. V., 1998. Pattern of bioindication of pollution of freshwater basing on results of histo-pathological analysis of mollusks. Biologiya vnutrennikh vod 3: 90–94 (in Russian).
Mack, R. N., D. Simberloff, W. M. Lonsdale, H. Ewans, M. Clout & F. A. Bazzaz, 2000. Biotic invasions: causes, epidemiology, global consequences, and control. Ecological Applications 10: 689–710.
Marguillier, S., F. Dehairs, G. van der Velde, B. Kelleher & S. Rajagopal, 1998. Initial results on the trophic relationships based on Corophium curvispinum in the Rhine River traced by stable isotopes. In Nienhuis P. H. (eds), New Concepts for Sustainable Management of River Basins. The Netherlands, Backhuys Publishers, Leiden: 171–177.
Martynov, A. V., 1932. To study of freshwater fauna of Black Sea coast of Caucasus. I. Amphipoda. Proceedings of Zoological Institute of Academy of sciences of USSR (Trudy Zoologicheskogo instituta Akademii nauk SSSR) 1: 73–98 (in Russian).
Mekhanikova, I. V., 2000. Morphological and ecological adaptation of baikalian amphipod Gmelinoides fasciatus to conditions of different aquatic ecosystems. Investigations of aquatic ecosystems of eastern Siberia. Proceedings of Biological and soil faculty of Irkutsk State university (Issledovaniya vodnikh ecosystem Sibiri. Trudy Biologo-pochvennogo facul’teta Irkutskogo gosudarstvennogo universiteta) 3: 104–114 (in Russian).
Mitskevich, O. I., 1981. Feeding on acclimatizated gammarids by fish in Lake Otradnoe. Proceedings of State Research Institute on Lake and River Fisheries (Trudy Gosudarstvennogo nauchno-issledovatel’skogo instituta ozernogo i rechnogo khozyaistva) 173: 71–76 (in Russian).
Mordukhai-Boltovskoi, F. D., 1960. Caspian Fauna in Azov and Black Seas Basin. Izdatelstvo Akademii nauk USSR, Moskva, Leningrad (in Russian).
Mordukhai-Boltovskoi, F. D. & Chirkova E. N., 1971. About distribution of Baikalian amphipod Gmelinoides fasciatus (Stebbing) in Gor’kovsky Reservoir. Biologiya vnutrennikh vod. Informatsionnyi byulleten’ 9: 38–42 (in Russian).
Mordukhai-Boltovskoi, F. D. & N. A. Dzyuban, 1976. Changes in structure and distribution of fauna of the Volga River under anthropogenic influence. In Nikol’sky G. V., & N. V. Butorin (eds), Biological and Production Processes in the Volga River Basin (Biologicheskie i produktsionnye protsessy v basseyne Volgi). Nauka, Leningrad: 67–82 (in Russian).
Nilova, O. I., 1976. Some features of ecology and biology of Gmelinoides fasciatus Stebb., acclimatized in Lake Otradnoe in Leningrad Region. Proceedings of State Research Institute on Lake and River Fisheries (Trudy Gosudarstvennogo nauchno-issledovatel’skogo instituta ozernogo i rechnogo khozyaistva) 110: 10–15 (in Russian).
Nyström, P., 1999. Ecological impact of introduced and native crayfish on freshwater communities: European perspectives. In Gherardi F. & D. M. Holdich (eds), Crayfish in Europe as Alien Species. How to make the best of a bad Situation. Brookfield, A.A. Balkema, Rotterdam: 63–85.
Odum, Y., 1975. Fundaments of ecology. Mir, Moskva (in Russian).
Olenin, S. & E. Leppäkoski, 1999. Non-native animals in the Baltic Sea: alteration of benthic habitats in coastal inlets and lagoons. Hydrobiologia 323: 233–243.
Panov, V.-E., 1996. Establishment of the Baikalian endemic amphipod Gmelinoides fasciatus Stebb. in Lake Ladoga. Hydrobiologia 322: 187–192.
Panov, V. E. & N. A. Berezina, 2002. Invasion history, biology and impacts of the Baikalian amphipod Gmelinoides fasciatus. In Leppäkoski E., S. Gollasch & S. Olenin (eds), Invasive Aquatic Species of Europe. Distribution, Impacts and Management. Kluwer Academic Publishers, Dordrecht, Boston, London: 96–103.
Panov, V. E., T. Timm & H. Timm, 2000. Current status of an introduced Baikalian amphipod Gmelinoides fasciatus Stebbing, in the littoral communities of Lake Peipsi. Proceedings of Estonian Academy of Sciences. Biology Ecology 49: 71–80.
Pienimaki, M., M. Helavuori & E. Leppäkoski, 2004. First findings of the North American amphipod Gammarus tigrinus Sexton, 1939 along the Finnish coast. Memoranda Societatis pro Fauna et Flora Fennica 80: 17–19.
Pirozhnikov, P. L. & T. I. Ioffe, 1974. Enrichment of Food Base for Fish in Reservoirs of USSR Through Acclimatization of Invertebrates. Izdatel’stvo Gosudarstvennogo nauchno-issledovatel’skogo instituta ozernogo i rechnogo khozyaistva, Leningrad (in Russian).
Romanova, N. N., 1959. Survival of some Amphipoda under different salinities. Proceedings of All-Union research institute of Fisheries and Oceanography (Trudy vsesoyuznogo nauchno-issledovatel’skogo instituta rybnogo hozyaistva i okeanografii) 38: 277–291 (in Russian).
Ruiz, G. M., T. K. Rawlings, F. S. Dobbs, L. A. Drake, T. Mullady, A. Hug & R. R. Colwell, 2000. Global spread of microorganisms by ships. Nature 408: 49–50.
Safronov, G. P., 1993. Composition and ecology of the genus Gammarus Fabricius species of the southern part of East Siberia. PhD Thesis, Irkutsk (in Russian).
Savateeva, E. V., 1985. Results of introduction of Baikalian amphipods and Ponto-Caspian mysids in Lake Ilmen. Proceedings of State Research Institute on Lake and River Fisheries (Trudy Gosudarstvennogo nauchno-issledovatel’skogo instituta ozernogo i rechnogo khozyaistva) 232: 3–15 (in Russian).
Shakhmatova, R. A. & P. I. Antonov, 1988. Seasonal dynamics of biomass, density and production of gammarids of the Volga River. Proceedings of Gorky University. Terrestrial and aquatic ecosystems (Mezhvuzovskii sbornik trudov Gor’kovskogo Universiteta. Nazemnye i vodnie ecosistemy ) 11: 102–112 (in Russian).
Shcherbina, G. K., N. R. Arkhipova & A. I. Bakanov, 1997. On the changes in zoobenthos biological diversity of the Upper Volga River and Gor’kovsky reservoir. In Popchenko V. I., & E. A. Bychek (eds), Problems of Biological Diversity of Aquatic Organisms in the Volga River basin (Problemy biologicheskogo raznoobraziya vodnikh organizmov Povolzh’ya). Izdatel’stvo instituta ecologii Volzhskogo basseina, Tol’yatti: 108–114 (in Russian).
Sidorov, E. G., 1963. Acclimatization of invertebrates and parasitological factor. Proceedings of conference on acclimatization of animals in USSR (Materialy konferentsii po akklimatizatsii zhivotnykh v SSSR). Izdatel’stvo Akademii nauk Kazakhskoy SSR: 356–358 (in Russian).
Simberloff, D., 1981. Community effects of introduced species. In Nitecki M. H. (eds), Biotic Crises in Ecological and Evolutionary Time. Academic Press. New York: 53–81.
Skal’skaya, I. A., 1996. Structure of population of the Baikalian amphipod Gmelinoides fasciatus (Stebb.) in Rybinskoe Reservoir. Biologiya vnutrennikh vod. Informatsionnyi byulleten’ 99: 29–35 (in Russian).
Slyn’ko, Y.-V., L. G. Korneva, I. K. Rivier, K. H. Shcherbina, V. G. Papchenkov, M. I. Orlova & T. W. Therriault, 2002. Caspian-Volga-Baltic invasion corridor. In Leppäkoski E., S. Olenin, & S. Gollasch (eds), Invasive Aquatic Species of Europe. Distribution, Impacts and Management. Kluwer Academic Publishers, Dordrecht, Boston, London: 339–411.
Sushchenya, L.-M., 1975. Quantitative Characteristics of Feeding of Crustaceans. Nauka i technika, Minsk (in Russian).
Sushchenya, L.-M., V.-P. Semenchenko & V.-V. Vezhnovets, 1986. Biology and Production of Glacial Relict Crustaceans. Nauka and technika, Minsk (in Russian).
Timm, V. & T. Timm, 1993. The recent appearance of a Baikalian crustacean, Gmelinoides fasciatus (Stebbing, 1899) (Amphipoda, Gammaridae) in Lake Peipsi. Proceedings of Estonian Academy of Sciences. Biology 42: 144–153.
Tomilina, I. M., 2000. Ecological and toxicological characteristics of bottom sediments in aquatic ecosystems of the North-West Russia. PhD Thesis. Borok (in Russian).
Van den Brink, F. W. B., G. Van der Velde & A. Bij de Vaate, 1993. Ecological aspects, explosive range extension and impact of a mass invader, Corophium curvispinum Sars, 1895 (Crustacea: Amphipoda), in the Lower Rhine (The Netherlands). Oecologia 93: 224–232.
Van der Velde, G., S. Rajagopal, B. Kelleher, I. B. Musko, A. Bij de Vaate, 2000. Ecological impact of crustacean invaders: general considerations and examples from the Rhine River. In von Vaupel Klein, C. J. & F. R. Schram, (eds), The Biodiversity Crisis and Crustacea. Proceedings of the fourth International Crustacean congress. Crustacean Issues 3. Brookfield. Balkema. Rotterdam: 3–33.
Vershinin, N. V., 1967. Biology and distribution of Gmelinoides fasciatus Stebb. in Bratsky Reservoir. Zoologicheskyi Zhurnal 46(7): 1024–1030 (in Russian).
Volkov, V. V. & I. I. Potina, 1977. Distribution, sizes and fecundity of Gmelinoides fasciatus (Stebbing), acclimatized in Gorkovsky Reservoir. Fishery Studies of Inland Waters (Rybokhozyaistvennoe izuchenie vnutrennih vodoemov) 21: 18–21 (in Russian).
Westman, K., 2002. Alien crayfish n Europe: negative and positive impacts and interactions with native crayfish. In Leppäkoski E., S. Gollasch & S. Olenin (eds), Invasive Aquatic Species of Europe. Distribution, Impacts and Management. Kluwer Academic Publishers, Dordrecht, Boston, London: 76–95.
Williamson, M. H. & A. Fitter, 1996. The character of successful invaders. Biological conservation 78: 163–172.
Witt, J. D. S., P. D. N. Hebert & W. B. Morton, 1997. Echinogammarus ischnus: another crustacean invader in the Laurentian Great Lakes basin. Canadian Journal of Fisheries and Aquatic Sciences 54: 264–268.
Zadoenko, I. N., O. A. Leis & V. F. Grigor’ev, 1985. Results and perspectives of acclimatization of the Baikalian amphipods in water bodies of the USSR. Proceedings of State Research Institute on Lake and River Fisheries (Trudy Gosudarstvennogo nauchno-issledovatel’skogo instituta ozernogo i rechnogo khozyaistva) 232: 30–34 (in Russian).
Zhadin, V. I., 1964. Malacostracan crustaceans of the Oka River according to data of 1959. Procceedings of Zoological Institute of Academy of sciences of USSR (Trudy Zoologicheskogo instituta Akademii nauk SSSR) 32: 149–154 (in Russian).
Acknowledgements
I thank the three anonymous referees for their valuable comments and constructive criticism and Tat’yana Platonova for editing the text and Dr. Elizabeth Cook for English revision. The study was supported by grants from the Russian Academy of Sciences Program on Biodiversity Conservation and the President’s grant “Russian Scientific Schools” (# 5577.2006.4).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berezina, N.A. Invasions of alien amphipods (Amphipoda: Gammaridea) in aquatic ecosystems of North-Western Russia: pathways and consequences. Hydrobiologia 590, 15–29 (2007). https://doi.org/10.1007/s10750-007-0753-z
Issue Date:
DOI: https://doi.org/10.1007/s10750-007-0753-z