Abstract
Freshwater pearl mussels (Margartifera margaritifera L.) are among the most critically threatened freshwater bivalves worldwide. The pearl mussel simultaneously fulfils criteria of indicator, flagship, keystone and umbrella species and can thus be considered an ideal target species for the process conservation of aquatic ecosystem functioning. The development of conservation strategies for freshwater pearl mussels and for other bivalve species faces many challenges, including the selection of priority populations for conservation and strategic decisions on habitat restoration and/or captive breeding. This article summarises the current information about the species’ systematics and phylogeny, its distribution and status as well as about its life history strategy and genetic population structure. Based on this information, integrative conservation strategies for freshwater mollusc species which combine genetic and ecological information are discussed. Holistic conservation strategies for pearl mussels require the integration of Conservation Genetics and Conservation Ecology actions at various spatial scales, from the individual and population level to global biodiversity conservation strategies. The availability of high resolution genetic markers for the species and the knowledge of the critical stages in the life cycle, particularly of the most sensitive post-parasitic phase, are important prerequisites for conservation. Effective adaptive conservation management also requires an evaluation of previous actions and management decisions. As with other freshwater bivalves, an integrative conservation approach that identifies and sustains ecological processes and evolutionary lineages is urgently needed to protect and manage freshwater pearl mussel diversity. Such research is important for the conservation of free-living populations, as well as for artificial culturing and breeding techniques, which have recently been or which are currently being established for freshwater pearl mussels in several countries.
Similar content being viewed by others
Introduction
Molluscs are an extremely diverse group of animals with more living species than birds, mammals, reptiles, amphibians and fishes combined (Lydeard & Lindberg, 2003). Thus, they are an important part of the overall biodiversity. Many of the molluscs have important functions in ecosystems.
The global decline of nonmarine molluscs is causing increasing concern (Lydeard et al., 2004). In particular, freshwater bivalve molluscs have shown severe declines during the last decade with many species now facing extinction. Freshwater mussels are probably the most endangered groups of animals (e.g. Bogan, 1993, 1998, 2008; Williams et al., 1993; Neves et al., 1997; Strayer et al., 2004). Given the high biomass and the high original abundances (hundreds of mussels per square metre) and thus the important roles of bivalve molluscs in particle processing, nutrient release, and sediment mixing (for review see Vaughn & Hakenkamp, 2001), the decline of mussel populations can have manifold implications on the functioning of aquatic ecosystems (Howard & Cuffey, 2006). Despite their importance, there is often a lack of knowledge about their complex biology, which connects the processes that influence their rapid declines.
One example is the freshwater pearl mussel (Margaritifera margaritifera L.), a highly threatened long-lived bivalve occuring in cool running waters of the Holarctic region. Some authors even consider it to be one of the most endangered freshwater mussels in the world (Machordom et al., 2003). About one century ago, freshwater pearl mussels still occurred in high densities, often covering the stream bottom in several layers (Israel, 1913). There had been an estimated decline of more than 90% in European populations by the 1990s (Bauer, 1988), a trend that has obviously continued or even increased. The current main concern is the lack of juvenile reproduction in most European pearl mussel populations.
Direct threats for adult mussels such as pearl harvesting, predation by muskrats, alien crayfish and eel (potentially feeding on juvenile mussels) have limited local influence and cannot explain the species’ global decline. Instead, indirect effects connected with anthropogenic perturbations such as habitat degradation, alteration and fragmentation are probably the most important factors for decline. A lack or decline of host fish populations and a series of additional interferences with the chemistry, biology, hydrology and geomorphology of streams may also have contributed to the current imperilment of pearl mussels.
Most European pearl mussel populations have lacked successful reproduction for 30–50 years, and in many cases their original distribution has dramatically receded. Thus, formerly dense and connected populations have often become fragmented and reproductively isolated remnant and island populations. However, a great potential for recovery is offered by the longevity of this species, i.e. a lifespan of more than 100 years (Bauer, 1992), together with the high reproductive potential that adult pearl mussels have, even in polluted rivers and at extreme old age.
Early conservation efforts have most often focussed on the effects of abiotic habitat factors on species (autecology) and on the complex relationships between species (synecology) with the intention of giving detailed descriptions of the species’ habitat requirements. Conservation planning has tended to focus more on pattern (representation) than process (persistence) and, for the former, has emphasised species, community or ecosystem diversity over genetic diversity (Moritz, 2002).
More recent conservation approaches have shown that ecological studies can greatly benefit from a combination with genetic studies. Genetic investigations into the extent and organisation of genetic diversity in populations and its spatio-temporal dynamics are a powerful tool to suggest sustainable conservation strategies. In particular, small and isolated populations can suffer from the effects of genetic drift and the loss of genetic variability, which contribute to inbreeding and rapid extinctions of such populations (extinction vortex). Recovery of small populations may be exacerbated by reduced fitness at low population densities (Allee effects) and stochastic factors. In addition, thorough ecological investigations are needed to reveal the specific requirements that must be fulfilled in the habitat during all life stages of the species. Both ecological and genetic reasons alone can lead to extinctions of populations, and the interaction of ecological and genetic factors may determine the dynamics, local occurrence or extinction of mussel populations. New research disciplines of Conservation Ecology and Conservation Genetics address these questions. The conservation of biodiversity between and within species have become priority goals, thus retaining the evolutionary potential for adaptation to future changes in the environment.
As with other freshwater bivalves, an integrative conservation approach that identifies and sustains ecological processes and evolutionary lineages is urgently needed to protect and manage freshwater pearl mussel diversity. Such research is important for the conservation of free-living populations, as well as for the development of artificial culturing and breeding techniques, which have recently been or which are currently being established for freshwater pearl mussels in several countries. The objective of this review is to summarise information on the systematics and phylogeny, the distribution and status, the life history strategy and the genetic population structure of pearl mussels, and to discuss integrative conservation strategies for freshwater mollusc species which combine genetic and ecological information, using the freshwater pearl mussel (Margaritifera margaritifera) as an example.
Systematics and phylogeny
Freshwater mussels and clams are members of the class Bivalvia within the phylum Mollusca. The large freshwater bivalves belong to the order Unionoida (=naiads, Unionacea) and had evolved from an as yet unidentified marine group by at least the Triassic (Watters, 2001). Bivalves of the order Unionoida are a diverse group of freshwater organisms (about 175 genera) with a broad distribution that currently includes all continents except Antarctica (Haas, 1969a; Roe & Hoeh, 2003). The Unionoida nominally include two superfamilies, the Etherioidea and Unionoidea, distinguished by larval forms (Parodiz & Bonetto, 1963; Haas, 1969b; Heard & Gluckert, 1970; Davis & Fuller, 1981; Boss, 1982). The Etherioidea (Muteloidea), with lasidia larvae, includes the Etheriidae (Africa, South America) and Iridinidae (Africa). The Unionoidea, with glochidia larvae, include the Hyriidae (Australasia, South America), the Unionidae (Africa, Eurasia, India, North America) and the family Margaritiferidae (Eurasia, North America), which is considered to be a basal and primitive clade within the Unionoidea (Haas, 1969a; Smith & Wall, 1985; Smith, 2001).
In his revised classification of the Margaritiferidae based on conchological, anatomical, biological and ecological characters, Smith (2001) proposes 12 margaritiferid species and suggests a classification into the three genera Pseudunio (five species), Margaritinopsis (six species) and Margaritifera, with Margaritifera margaritifera being the only species of the genus. Recent investigations into the phylogenetic relationships of the Margaritiferidae based on molecular data, however, indicate that the group is in need of revision since the genus is not monophyletic and the taxonomy by Smith (2001) is not supported (Huff et al., 2004).
Hypotheses on the historical geographical dispersal of the Margaritiferidae conflict. Some authors assume that early dates of wide clade distribution suggest the break-up of the supercontinent Pangea as the cause of dispersal (Davis & Fuller, 1981; Smith, 2001), but it is alternatively suggested that colonisation might have occurred more recently when salmonid hosts released juvenile margaritiferids onto the North American continent (Machordom et al., 2003).
Based on recently sequenced COI data, two monophyletic clades have been identified within the Margaritiferidae: one including M. margaritifera, M. dahurica, M. falcata and M. laevis, and a second clade comprising M. auricularia and M. marocana, which has recently been recognised as a valid species (Araujo et al., 2009). In Europe, two extant species of pearl mussels are described, M. (Pseudunio) auricularia (Spengler, 1793), an almost extinct species occurring in Southern Europe, and M. margaritifera (L., 1758), both of which encompass a number of contentious or uncertain taxa of lesser rank. Especially the taxonomic status of the last remaining population of the critically endangered Irish hardwater species/subspecies M. (m.) durrovensis (Phillips, 1928) has been a matter of several scientific discussions (e.g. Chesney et al., 1993; Moorkens & Costello, 1994; Chesney & Oliver, 1998). Recent investigations support the view that it is an ecophenotype of M. margaritifera (Machordom et al., 2003). It is often stated that the systematics of European naiads have been a battlefield for very different opinions with few other groups having been subject to so many controversies on the number of species involved, their distinction and their phylogenetic relationships (Nagel et al., 1998). With M. margaritifera, a number of disputed and uncertain taxa of subspecies rank have arisen due to the wide range of shell shapes and textures observed among populations (Chesney & Oliver, 1998) demonstrating the need for thorough genetic investigations instead of an over-reliance on highly variable morphological shell characters.
Distribution and population structure
The freshwater pearl mussel (Margaritifera margaritifera L.) is a Holarctic species which is distributed from the arctic and temperate regions of western Russia, westwards through Europe to the northeastern seaboard of North America (Jungbluth et al., 1985). With only a few exceptions, pearl mussels are exclusively found in rivers and streams which are extremely low in lime and nutrients.
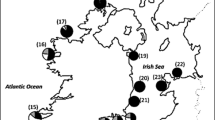
The most accurate and detailed reviews of the current distribution and population status of European freshwater pearl mussels are available from Sachteleben et al. (2004), Young et al. (2001) and Araujo & Ramos (2000). However, all of them lack some information due to recent rediscoveries, declines and extinctions of some populations. Figure 1 and Table 1 attempt to provide information on the current distribution and populations of pearl mussels considering the most accurate data available, based on recent publications, a series of personal communications in the years 2005–2009, and personal survey work carried out during the years 2003 to 2009. It has to be noted, however, that no reliable information is available for certain geographical regions due to a lack of recent survey work, as indicated in Fig. 1 and Table 1. In North America, M. margaritifera occurs on the Atlantic coast from Newfoundland, Canada, down to Delaware and Pennsylvania, USA and westwards to the Appalachian mountains (Ziuganov et al., 1994) but the current status of populations seems to be unknown.
Pearl mussel distribution and populations in Europe. Green circles indicate secure current M. margaritifera populations with significant percentage (>20%) of juveniles younger than 20 years; white circles indicate secure populations from recent surveys without proof of sufficient juvenile recruitment; the blue triangles represent the probably last remaining M. auricularia populations in Europe. The black line refers to the southern distribution limit of M. margaritifera in Europe. Note that single spots can refer to population units comprising more than one population and that the actual numbers of populations remain unclear for some geographical regions, indicated by question marks
In Europe, the species was originally widespread and formed the basis for significant pearl fisheries. At present, the largest European pearl mussel populations with several million individuals and an intact age structure occur in Russian rivers of the Kola peninsula (Ziuganov et al., 2001). Large populations are also reported from Scandinavia and the British Isles, with Scotland still holding a large number of important populations (Young & Williams, 1983). Pearl mussel distribution in the south of the species’ range on the Iberian peninsula was originally considered to be limited to a few small populations in Northern Spain (Bauer, 1986), until important and reproductively active populations have recently been rediscovered in Portugal (Reis, 2003) and in Galicia in North-West Spain (Outeiro et al., 2008; San Miguel, pers. comm.).
The largest central European pearl mussel populations are found in the drainages of the Elbe, the Danube, the Weser, the Main/Rhine and the Maas, comprising the countries of Germany, the Czech Republic, Austria, Belgium and Luxembourg. In addition, a number of (usually small) populations still exist in France (Massiv Central, Arquitaine, Brittany) and in the Baltic States. Significant numbers and proportions of juveniles that justify a classification of the populations as sustainably “functional” only occur in a handful of European populations in the countries of Germany (Lutter), the Czech Republic (Blanice), Portugal (Douro tributaries), Scotland (several rivers), Ireland (Western populations), Northern Scandinavia (e.g. Pikku-Luiro) and Russia (e.g. Varzuga drainage). A number of additional populations show limited reproduction which will probably not be enough to secure their current status. The vast majority of European populations are extremely overaged, with the youngest individuals usually being 30–50 years old and with no juvenile mussels detectable during intensive surveys (Fig. 2).
Length–frequency distributions of two pearl mussel populations, one of them considered to be functional (PI, Northern Lapland), and one overaged population (WB, central Europe) showing a distinct lack of juvenile reproduction. Mussels <2.5 cm cannot be reliably counted in field surveys. Note that pearl mussels show asymptotic growth and that interruptions of juvenile recruitment even happened in the functional population
The global decline of freshwater pearl mussel populations in the last 50 years has attracted much concern from national and international conservation organisations (Araujo & Ramos, 2000; Strayer et al., 2004). They are currently listed in the European Habitats & Species Directive Annexes II and V, the Bern Convention Annex 3, and are a priority species in many European Biodiversity Action plans.
Within streams, pearl mussels often occur in non-random, clumped distribution patterns (Hastie et al., 2000). An understanding of mussel distribution and abundance within stream ecosystems is crucial for their conservation (Strayer, 2008).
Life history strategy
Like all other large freshwater mussels of the order Unionoida, Margaritifera margaritifera is characterised by a semi-infaunal mode of life in its adult phase, being partly buried into the stream substratum. Adult pearl mussels can actively move by pumping haemolymph into their foot, but they are very sessile in comparison with other naiads.
Freshwater pearl mussels are among the longest-lived invertebrates known, frequently reaching ages of more than 100 years (Bauer, 1992) and a maximum length of 15 cm. The maximum age reached is highly variable among populations and seems to primarily depend on growth rates. Populations tend to be faster growing and shorter lived in the southern part of their range with Spanish populations only attaining 35 years (Miguel et al., 2004), whereas pearl mussels in cooler Scandinavian climates can exceed ages of 200 years (Mutvei & Westermark, 2001).
As with all unionoid mussels, freshwater pearl mussels have a complex life cycle (Fig. 3). As in other freshwater bivalves, the sexes of M. margaritifera are usually separate but females were observed to become hermaphrodites at low population densities (Bauer, 1987a). The complex reproductive strategy of freshwater pearl mussels is marked by a high fertility resulting in a single female producing several million larvae (glochidia) per year (Young & Williams, 1984). In mid- to late summer the glochidia are discharged into the river. A recent study estimated peak releases up to 441 million glochidia per day for a Scottish population (Hastie & Young, 2003b). The proportion of adults producing glochidia is relatively high even in sparse populations (Young & Williams, 1983; Schmidt & Wenz, 2000; Schmidt & Wenz, 2001; Hastie & Young, 2003b), and reduced fecundity does not seem to be the limiting factor preventing juvenile recruitment in most pearl mussel populations.
Freshwater pearl mussel populations are highly dependent on viable host fish populations. In the first stage of the life-cycle after their release, the glochidia of M. margaritifera must be inhaled by a suitable host fish, where they live encysted as obligate gill-parasites for a period of up to 10 months (Bauer, 1994). Glochidia only remain infective for a few days and over short distances downstream of the sites from where they are released (Jansen et al., 2001). Only sea trout (Salmo trutta f. trutta), brown trout (Salmo trutta f. fario) and Atlantic salmon (Salmo salar) are known to host complete metamorphosis in Europe, where they are the only native host species (Young & Williams, 1984). Salmon appear to be the main hosts in Nova Scotia (Cunjak & McGladdery, 1991) and Russia (Ziuganov et al., 1994). In central Europe, brown trout are reported to be the preferred or the only available hosts (Bauer 1987b, c; Wächtler et al., 2001; Geist et al., 2006). Brook trout (Salvelinus fontinalis) appears to be an important host fish for pearl mussel in North America but is an unsuitable host in Europe (Ziuganov et al., 1994). Glochidial rejection is not only limited to non-host fish. Many fish hosts become progressively resistant to glochidial infection (Young & Williams, 1984; Bauer & Vogel, 1987; Ziuganov et al., 1994). It remains uncertain whether pearl mussels can be considered to be parasites only, as their host fish may benefit from the reduced suspended organic material in river water by filter-feeding by the mussels. In addition, mussel beds can also provide important microhabitats for juvenile salmonids and the aquatic invertebrates upon which they feed (Hastie & Cosgrove, 2001). Ziuganov & Nezlin (1988) consider the relationship between mussel and fish to be a variety of symbiosis-protocooperation. Links between the local decline of fish hosts and the decline in mussel populations have been suggested (e.g. Hastie & Cosgrove, 2001). Comparative investigations of host fish densities and biomass between functional (i.e. recently reproducing) and non-functional pearl mussel populations indicate, however, that host fish density is probably sufficient in most areas (Bauer et al., 1991; Geist et al., 2006).
During their post-parasitical phase, juvenile pearl mussels bury themselves into the stream sediments for a period of 5 years, where they depend upon a stable substrate with high sediment quality and intense exchange between free-flowing water and interstitial water (Buddensiek et al., 1993; Geist, 1999a, b; Geist & Auerswald, 2007). There is consensus among researchers that the post-parasitic stage can be considered the most critical stage in the life cycle of the pearl mussel (Buddensiek et al., 1993; Geist & Auerswald, 2007 and references therein). The huge losses involved in this extraordinary life cycle make the freshwater pearl mussel particularly vulnerable to adverse conditions (Skinner et al., 2003).
Genetic population structure and mussel propagation
Based on COI and 16S rRNA sequences, two closely related mitochondrial lineages have been identified in Margaritifera margaritifera: a northern lineage extending from Ireland to the Kola peninsula, and a second cluster distributed from Ireland to the Iberian peninsula (Machordom et al., 2003). The same study revealed a close relation of specimens from both sides of the Atlantic. In contrast to allozyme and mitochondrial variability, analyses of microsatellites revealed high degrees of population structure and very different levels of genetic diversity among European populations (Geist et al., 2003; Geist & Kuehn, 2005; Bouza et al., 2007; Geist & Kuehn, 2008; Geist et al., 2009). Genetic variability as measured by allelic richness and heterozygosity levels appears to be the highest in the north-east of the species distribution range which can be explained by the species life history strategy and by the lesser extent of habitat destruction in these areas (Geist & Kuehn, 2008). The strong genetic differentiation of pearl mussel populations, even within small geographical scales, may have been strongly enhanced by the effects of genetic drift, particularly in southern and central Europe (Geist & Kuehn, 2005; Bouza et al., 2007). The geographical distribution of genetic diversity in pearl mussel seems to be inversely related to that of its host fish, brown trout (Geist & Kuehn, 2008)—a phenomenon which can be explained by differences in the life history strategies and the ecological niches of the two species. Genetic criteria gained from the study of molecular markers can be very useful for the identification of Conservation Units (CUs) and for the selection of candidate populations to be given priority for conservation (e.g. Petit et al., 1998). Based on a comparison of the heterozygosity contribution of individual populations to average heterozygosity levels, genetically determined priority populations for conservation have been identified for pearl mussels and for their host fishes in different geographical regions (Geist & Kuehn, 2005, 2008; Geist et al., 2009).
The propagation and culture of endangered mussel populations and species to augment population sizes and to reintroduce species and populations to sites within their historical ranges is often recommended in species recovery plans (Barnhart, 2006). In the case of pearl mussels, the availability of culturing techniques has been considered a breakthrough in their conservation (Preston et al., 2007). Preservation of genetic diversity requires robust genetic analyses of source populations. At the same time, artificial selection and other genetic hazards (e.g. loss of genetic variation) affecting adaptive traits of progeny subsequently released to the wild can be minimised as per ten suggested genetic guidelines for captive propagation of freshwater mussels (Jones et al., 2006). Owing to the pronounced population structure and the wide range of genetic diversity levels among different pearl mussel populations, practical management guidelines founded in genetic data must be population specific. The sampling of gravid mussels for supportive breeding and culturing is typically limited to small numbers of individuals, particularly in small remnant populations. The selection effect during sampling and the specific environmental conditions during the culturing process can result in loss of genetic variation (due to genetic drift and/or selection) in the offspring generation and alter the gene pool of the source population upon release of the juveniles. In contrast to many other species, pearl mussels have an extremely long reproductive life span and these deleterious effects on the gene pool can be compensated by using different gravid parent mussels in different years or by genetic-aided selection of parent individuals. Both practices require appropriate tagging of adult specimens for reliable identification. In general, a cumulatively large number of gravid females sampled from different locations is likely to result in increased genetic variability of the offspring. Tagging of released propagated juvenile mussels for subsequent identification is also advisable.
Conservation strategies
The development of sustainable conservation strategies for endangered freshwater pearl mussels and other aquatic organisms is complex, and, therefore, several spatial and temporal issues are important. In order to be successful, conservation efforts must be focussed on preserving the processes of life (Bowen, 1999). The freshwater pearl mussel is a species which offers great potential to meet these challenges and to discuss sustainable conservation strategies in the context of Conservation Genetics and Ecology. Despite the fact that urgent conservation recommendations are needed to maintain the last remaining European pearl mussel populations, conservation strategies must be based upon scientific facts. Development of conservation strategies unique to mussels must be grounded in an understanding of their life histories, population genetic structure and population dynamics (Jones et al., 2006). Ecological and genetic management objectives and actions can differ but should be integrated at different levels of management action (Table 2).
Research and conservation address problems at various levels of organisation: problems at the individual and population level, problems at the species level in the entire range, problems of community and ecosystem diversity, as well as problems connected with the overall goal of sustaining global biodiversity.
Aspects of conservation on the individual and population level
As a first step on the individual and population level, a thorough understanding of the autecology and habitat requirements of pearl mussels is needed to be able to evaluate the current habitat quality, including the assessment of anthropogenic impacts. Different habitat requirements must be met during all phases of the species’ complex life cycle, and potential adaptive differences between populations and genetic variability in individuals and populations must also be considered to address these questions thoroughly.
Almost all European pearl mussel populations, even those in nutrient enriched streams or in sparse populations, seem to still have a high proportion of adults producing glochidia on a normal level (e.g. Young & Williams, 1983; Schmidt & Wenz, 2000; Schmidt & Wenz, 2001; Hastie & Young, 2003b). Thus, problems with this initial phase in the life cycle do not seem to be the primary reason for the serious population declines. Given the high reproductive potential of pearl mussels and the fact that no reduction in fecundity of old mussels has been observed, even small and overaged populations that have lacked reproduction for many years can potentially recover after habitat restoration or through supportive breeding measures. The observed metapopulation structure (Geist & Kuehn, 2005) and investigations into the demographic structure of viable Scandinavian pearl mussel populations (Fig. 2) suggest that a temporal lack of juvenile recruitment over some years can be tolerated or even normal in long-lived and healthy populations.
Freshwater pearl mussels are excellent indicators for the interaction of different environmental habitat compartments due to their complex life cycle. Their conservation cannot be viewed separately from that of their host fish, and thus a synecological perspective on the interactions between species in the ecosystem is required. In a previous research, there was a distinct lack of field data on fish communities and of adequately researched host fish densities in pearl mussel streams (Skinner et al., 2003). The suspicion that effects of acidification in the oligotrophic, poorly buffered pearl mussel streams may have caused extinctions of host fish populations, and a poor knowledge about the interrelation of host stock sizes and the reproductive success of mussels (Chesney & Oliver, 1998) demanded that sound and quantitative investigations be carried out in this field. Indeed, the results of Geist et al. (2006) showed that a complete lack of host fish or severely disturbed host fish populations can occur in specific pearl mussel streams, and these alone are a sufficient explanation for the lack of juvenile recruitment in these populations. However, this study also revealed that the size and composition of host fish populations appears to be limiting for pearl mussel reproduction only in a small number of streams in certain geographical regions. Even comparatively small host fish populations seem to be sufficient to support large pearl mussel populations if habitat conditions during other phases of the life cycle (e.g. substratum quality and stability, and the survival rate during the post-parasitical phase) are optimal. This example clearly demonstrates the need for interdisciplinary research, as one phenomenon—the decline of pearl mussel populations—can be attributed to different and multiple reasons in different geographical regions.
Several studies suggest that the survival rates of pearl mussels during the post-parasitical phase are crucial and the key issue linked with the lack of juvenile recruitment in most populations (e.g. Buddensiek et al., 1993; Geist & Auerswald, 2007). The comparatively high host fish densities and intact age structures of host fish populations found for most pearl mussel streams (Bauer et al., 1991; Geist et al., 2006), and the observed poor sediment quality and low rates of exchange between the free water body and the interstitial water in many European pearl mussel streams support this view. During their long post-parasitical phase in which pearl mussel live buried into the stream substratum for usually 5 years, pearl mussels depend on a permanently well-oxygenated and stable substrate. In fact, studies into sediment microhabitats of pearl mussel populations at sites with high rates of juvenile recruitment all showed low percentages of fine sediments, high redox potentials and no or only small differences in the chemistry of water taken from different depths of the interstitial zone and from the free water (Geist & Auerswald, 2007). These criteria are rarely fulfilled in central European populations and deserve special attention. In addition, the relationship between the river flow regime (i.e. the incidence and intensity of floods) and juvenile mussel survival is likely to be of crucial importance in the light of climate change. Substrate factors probably also closely correlate with the productivity and food availability for juvenile pearl mussels, a field which is still poorly investigated and understood.
Conservation and management strategies on a population level when there are certain habitat deficiencies can be overcome by artificial culturing and breeding techniques (e.g. Barnhart, 2006). For instance, inadequate host fish populations can be bridged by artificial infection of autochthonous host fish, the infection of host fish in hatcheries and the release of infected fish shortly before drop-off of glochidia, or by directly releasing juvenile mussels from artificially infected and farm-reared host fish. Similarly, the culturing of juvenile mussels in cages or artificial bypass-channels with high sediment quality can reduce mortality rates during the post-parasitical phase if sediment quality or stability is not sufficient in the main stream. The feasibility of culturing M. margaritifera as a conservation tool has been studied by Buddensiek (1995), Hastie & Young (2003a), and promising results in this field are reported from the Czech Republic (J. Hruška, pers. comm.), Germany (M. Lange, pers. comm.), the United Kingdom (Preston et al., 2007) and Luxembourg (F. Thielen, pers. comm.). However, such conservation strategies are (semi-)artificial and can only be carried out for a small selection of populations. They should be seen as an important but temporary emergency measure to rescue and maintain genetically unique populations and their variability until the natural habitat can be restored. The example of the river Lutter in Northern Germany clearly illustrates that the reduction of anthropogenic sand and silt loads to a natural level can ultimately induce successful recruitment of freshwater pearl mussels in the wild and enhance the population status of other substratum-dependent species such as minnow (Phoxinus phoxinus), bullhead (Cottus gobio) and green gomphid (Ophiogomphus cecilia) (Altmüller & Dettmer, 2006).
As different levels of individual or population genetic variability (e.g. heterozygosity, allelic richness etc.) are often correlated with fitness parameters and the ability to adapt to changes in the environment (e.g. Reed & Frankham, 2003), an evaluation of these genetic parameters on an individual and population level can help to develop sustainable conservation, breeding and culturing strategies for the species, and to avoid genetic bottlenecks and founder effects (Geist & Kuehn, 2005). The installation of breeding programmes on a genetic basis should, therefore, consider measures to maintain the genetic identity of evolutionary significant units (ESUs) and conservation units (CUs) on the one hand, and reduce the effects of genetic stochasticity on small populations on the other hand. Density dependent effects (Allee effects) may contribute to reduced fitness and chances for the recovery of small populations both for genetic and ecological reasons. In general, careful evaluation of genetic relationships and habitat suitability is necessary before carrying out stocking activities with freshwater mussels (Geist & Schmidt, 2004; Jones et al., 2006).
An improved understanding of ecology and ecological habitat changes is essential for managing the genetic diversity of threatened and endangered species properly. Genetic studies can in turn be beneficial for ecological studies. This approach, landscape genetics, promises to facilitate our understanding of how geographical and environmental features structure genetic variation at both the population and individual levels, and has implications for ecology, evolution and conservation biology (Manel et al., 2003). In some cases, different conservation management strategies can be deduced from results of different scientific approaches. This can be illustrated for the practical management issue of whether it is a useful conservation measure to collect mussels from small populations and to put them together into aggregations. From an ecological point of view, a dispersed population structure of pearl mussels reduces the risk of extinction due to local catastrophes. It also largely increases the number of potential host-fish infections and thus has positive effects on the number and dispersal of juvenile pearl mussels (Geist et al., 2006). It also prevents the mussels from being exposed to stress due to their translocation. On the other hand, from the genetic point of view, the opposite strategy of putting mussels from small populations together in one group may be suggested to avoid selfing, the effects of inbreeding and genetic stochasticity on small populations. In addition, monitoring of population size becomes easier if mussels are being aggregated. The conflict of giving priority to genetic or ecological arguments can typically only be resolved on the basis of specific populations, and requires a careful balancing of arguments.
Monitoring, dating and assessment of past changes in the environment can be a promising approach for detecting, identifying and investigating the influence of environmental factors that can explain the species’ dramatic declines in specific populations. Long-lived adult pearl mussels themselves with their tree-like annual shell growth increments can be used as an environmental or physiological long-term archives (e.g. Mutvei & Westermark, 2001; Geist et al., 2005). Patterns of stable carbon δ13C signatures in annual shell carbonate growth increments were found to be a marker for metabolic activity, as mussels exposed to identical environmental conditions revealed different individual signature patterns extending over several years (Geist et al., 2005). Linking these patterns with biological processes of mussel physiology and growth can reveal insights into the individual performance and overall fitness of mussels. This methodology of mussel shell analyses may also be useful for other mollusc species and for annual analyses of the temporal dynamics of environmental variables, such as acidification, eutrophication or pollution effects which are similarly recorded and preserved in mussel shell long-term archives (e.g. Carell et al., 1987; Lindh et al., 1988; Mutvei & Westermark, 2001). As demonstrated by Geist et al. (2005), a combination of stable carbon isotope analyses with stable nitrogen isotope analyses of mussel tissues and potential food sources improve our understanding of physiology and food sources for pearl mussels. An understanding of the dietary requirements of pearl mussels at different stages of their development is crucial for both captive breeding and restoration of natural habitats.
Aspects of conservation on the species level
In addition to regional attempts to protect and support individual pearl mussel populations, it is essential to consider the species’ biodiversity on a more global scale. Conservation resources are limited. Thus, they require priority setting for populations within species and for biogeographic areas within regions, the incorporation of knowledge of evolutionary processes and the distribution of genetic diversity into conservation planning (Moritz, 2002). Characterisation of genetic variability plays a key role in defining strategies for species conservation which, by definition, seeks to protect a threatened gene pool.
As a first step on the species level, detailed survey work to map current populations and to assess their demography and current imperilment status is required. Recent suggestions for monitoring the freshwater pearl mussel are available from Young et al. (2003). Among these populations, priority populations for conservation can be selected by a combination of genetic and ecological methods. From the genetic perspective, conservation units (CUs) should be identified (Geist & Kuehn, 2005). The conservation goals attributed to the concept of CUs for freshwater pearl mussel populations involve maintaining genetic diversity in the species, combining concepts of minimum viable populations (Soulé, 1987; Nunney & Campbell 1993), evolutionary significant units (ESUs) (Moritz, 1994; Crandall et al., 2000), and management units (MUs) (Moritz, 1994). Ideally, genetic diversity should be separated into two dimensions, one concerned with neutral divergence, and the other with adaptive variation. Most recent conservation genetics research has focussed on the use of neutral genetic markers (Hedrick, 2004), which have been developed and applied in freshwater pearl mussel (Geist et al., 2003; Geist & Kuehn, 2005; Geist & Kuehn, 2008). In addition, coding mitochondrial markers are available for pearl mussels (Geist, 2002; Machordom et al., 2003; Huff et al., 2004; Araujo et al., 2009). The application of genetic markers for analysing population diversity and differentiation appears to be especially important among bivalve molluscs, as morphological features can largely depend on environmental variables (e.g. Johnson, 1970; Watters, 1994).
Ideally, no important populations should be omitted during investigation to be able to assess the contribution of each population to the species’ total diversity and differentiation. As most extant pearl mussel populations are small, critically endangered and strictly protected, negative impacts on the mussels must be excluded by using non-destructive DNA sampling techniques. For genetic analyses based on living individuals, the non-destructive techniques of haemolymph sampling (Geist & Kuehn, 2005) and of viscera swabbing (Henley et al., 2006) have been demonstrated to be most effective. Sampling of soft tissue (e.g. mantle clippling from living mussels) is highly subjective to the sampler’s experience and has been shown to result in increased mortality and regression of shell formation, at least in some mollusc species (Henley et al., 2006 and references therein).
In addition to the knowledge about the current genetic structure of extant populations, a better understanding of historical processes connected with the species’ phylogeny, phylogeography, colonisation and extinction patterns can be helpful for future conservation strategies and for selecting closely related source populations for reintroductions into extinct populations. Thus, it can be useful to additionally include samples from extinct populations into genetic studies. The analysis of shell DNA was demonstrated to be possible, but is more complicated than haemolymph or tissue DNA analyses, and certain precautions are necessary due to the low quantity and quality of shell DNA (Geist et al., 2008).
For selection of priority populations for conservation, the ecological aspects of habitat evaluation, eventually integrating an assessment of the chances for habitat restoration—including the landuse in the catchment—should be equally considered. This process is comparatively easy if conservation units comprise several populations with similar genetic composition. Under such circumstances, it appears to be reasonable to select priority populations with the most intact habitats by indirect means of pearl mussel population size, age structure, or direct means, e.g. sediment quality, host fish densities or landuse in the catchment area. Habitat dynamics, anthropogenic impacts and economic aspects should also be considered. Conservation strategies become more difficult when genetically unique populations with significant contribution to the species’ total diversity coincide with heavily disturbed habitats, a negative evaluation of ecological habitat parameters, e.g. in river catchment areas with intensive landuse. In such cases, captive propagation is often the only available short-term conservation option to rescue the gene pool since habitat restoration is very time consuming. In general, it is often discussed whether it is more reasonable to focus conservation approaches on single large or on several small populations, the so-called SLOSS-controversy (Simberloff & Abele, 1982). The results of genetic investigations on pearl mussels suggest a more complex discussion of this topic, since both small and large populations seem to considerably contribute to the species’ genetic diversity and differentiation (Geist & Kuehn, 2005).
In the next step after the selection of priority populations, strategies to maintain the genetic diversity of the priority populations are required to retain the species’ evolutionary potential. The most critical task from the conservation genetics point of view is the balancing between avoidance of inbreeding effects on the one hand and outbreeding effects on the other hand, a topic which is even more difficult for a species like the pearl mussel with facultative hermaphrodism. Maintaining genetic variability of pearl mussels to avoid the effects of genetic stochasticity on small populations is important and can include the re-establishement of gene flow between closely related populations, the so-called migration rescue (Lenormand, 2002). On the other hand, gene swamping between evolutionary significant units adapted to specific habitats can have deleterious genetic effects, the so-called migration meltdown (Ronce & Kirkpatrick, 2001). The conservation of multiple populations from each genetic cluster or conservation unit is advisable and should ideally include different catchments and geographical regions for reducing extinction risk.
As conservation actions to protect mussels must often be pursued without waiting for research to provide the final answers, adaptive management is suggested to be a useful tool (Strayer et al., 2004). Effective adaptive conservation management requires an evaluation of previous actions and management decisions. However, it also has to be considered that among long-lived and slow-growing species like freshwater pearl mussels, the time lags between a stressor (e.g. habitat loss or restoration) and the appearance of its effect (e.g. population collapse or rediscovery of juvenile recruitment) are long and can disguise the current status of populations and the effects causing the declines or recoveries. Thus, conservation actions without immediate positive effects on pearl mussels must be judged carefully, and the interactions with other species and the complete ecosystem should be given priority. The successful habitat restoration in the river Lutter with the significant effects on pearl mussel recruitment and improvement of the status of other endangered species (Altmüller & Dettmer, 2006) can serve as a valuable reference.
For the deduction of effective conservation strategies for European freshwater pearl mussels and for aquatic biodiversity conservation in general, an interdisciplinary approach integrating aspects of conservation genetics and ecology in large geographical ranges is needed, which—on a next level—also have to consider human dimensions to become sound management strategies.
Conservation of global biodiversity: Margaritifera margaritifera as a target species for aquatic biodiversity conservation
The monitoring and conservation management of biodiversity above species level is even more complex but also more important than that on the level of a single species. In particular, the points of how to define priority habitats and species associations are not free from personal opinions. Despite the fact that invertebrate species represent about 99% of animal diversity (Ponder & Lunney, 1999), and the fact that molluscs belong to the second-most diverse animal phylum in terms of numbers of described species (Lydeard et al., 2004), invertebrate and mollusc diversity is strongly under-represented in conservation research (Bouchet et al., 1999; Clark & May, 2002; Lydeard et al., 2004). Recently, 25 locations were identified as global hotspots for conservation prioritisation, and it was suggested that the limited conservation resources available should be put into these areas first (Myers, 2003). These hotspots were identified based on areas with high levels of species endemism in plants, mammals, birds, reptiles and amphibians, but invertebrate diversity is not even specifically mentioned. Such approaches of grossly disproportionate distribution of taxonomic effort towards vertebrates and higher plants (Gaston & May, 1992) remain questionable, since an Australian study showed that invertebrates can be strong predictors for conservation priorities for vertebrates, but not vice versa (Moritz et al., 2001). Diversity of freshwater bivalves across the main zoogeographic regions is extremely variable and was reported to not completely correspond to the standard zoogeographic regions (Bogan, 2008), making the selection of priority areas for conservation difficult.
It is often suggested to generally focus conservation efforts on indicator, flagship, umbrella or keystone species. Some species fulfil one or two of these conditions; some even none. The freshwater pearl mussel can be seen as an exception, as this species at least partly matches criteria involved in all of these concepts.
Margaritifera margaritifera can be seen as an indicator species, as it is a stenoecious species which is adapted to cool, oxygen-saturated running waters which are low in lime and nutrients. Pearl mussels are easy to identify and occur in a wide geographic range. They have a complex life cycle, they are long-lived and they are particularly sensitive to eutrophication and other changes in water quality. Although pearl mussels do not appear to be indicators for fish species richness in headwater regions (Geist et al., 2006), they are good indicators for the co-occurrence of specialised species: ecosystem health and functioning (e.g. nutrient cycles), and structural diversity, being important factors e.g. for their fish hosts and for a series of accessory species, such as lampreys and the larvae of ephemeropterans, trichopterans and plecopterans.
A conservation strategy for umbrella species is orientated towards providing sufficiently large areas for species with a wide home range, also bringing other species under that protection. The factors which control mussel populations can arise at various distances from the mussels (Strayer et al., 2004). While local conditions are undoubtedly important for mussels, more distant factors, such as geology and landuse in the catchment area, can have strong effects as well. With the new European Water Framework Directive in place, catchment level plans (river basin plans) are now required which may also be useful for better considering the catchment effects on pearl mussels and other species. Restoration of complete river catchments is probably the key to successful restoration of pearl mussels and other substratum-dependent species (Altmüller & Dettmer, 2006). In Addition, it seems that functional pearl mussel populations match a metapopulation model in many areas, implying positive effects of gene flow between subpopulations within evolutionary significant units of interconnected river systems (Geist & Kuehn, 2005). This largely depends on the existence of intact river systems without artificial barriers (e.g. man-made dams or sewage inputs) that hamper or prevent the migration of host fish vectors. Thus, pearl mussel conservation is a wide-ranging conservation approach, matching the ideas underlying the concept of an umbrella species, although extant pearl mussel populations are most often only limited to small patches in the headwaters of streams in most central European populations.
The freshwater pearl mussel has become a popular symbol and leading element of entire conservation campaigns, which is attributed to the concept of flagship species. Despite the fact that the species is not as charismatic as large vertebrates, the pearl mussel is identified with pristine and healthy stream ecosystems and has been used as a poster-animal, e.g. on stamps in Germany and the Czech Republic. The cultural and historical importance of the species producing valuable pearls may contribute to the symbolic character.
It has to be considered that single species management of flagships, umbrellas, endangered species and others can lead to the odd circumstance that their management conflicts with the management of other species, and that single species management of an indicator species by means of only supporting this species with semi-artificial measures is a self-contradiction (Simberloff, 1998). Conservation strategies addressed towards a rescue of sustainable pearl mussel populations will require habitat restoration and will also benefit its host fish and a series of similarly vulnerable but less popular species, which matches the idea of functional keystone species.
The concept of the keystone species suggests that certain species have impact on many others, often far beyond what might have been expected from consideration of their biomass or abundance. The original definition of ‘keystone’ has been expanded (Bond, 1993; Menge et al., 1994), and species that are not near the top of foodwebs have also been seen as keystones. Thus, the freshwater pearl mussel may ideally match the ideas behind the concept of keystone species. Changes of the physical structure of stream sediments by dense mussel populations, their effects on water clearance, light penetration, abundance of macrophytic plants and the resultant increase in aquatic organisms dependent on these structures for attachment, food or cover, are examples which illustrate that freshwater bivalves in general, and freshwater pearl mussels in particular can be viewed as keystone fauna of aquatic ecosystems, their presence greatly enhancing biodiversity and ecosystem functioning (e.g. Vaughn & Hakenkamp, 2001; Howard & Cuffey, 2006).
Given the suitability of pearl mussels as flagship species on the one hand, and their important ecological functions as indicator, keystone and umbrella species on the other hand, they can be seen as an ideal target species for practical conservation efforts in stream ecosystems.
In general, conservation priorities should move away from simple species- and habitat-orientated goals towards the idea of conserving the evolutionary process on which entire biodiversity depends.
Recommendations for future research
Future pearl mussel research on the individual and population level should particularly focus on the habitat requirements of juveniles during their post-parasitical phase, including studies on substratum quality, dynamics and their influences on the food webs. The use of stable isotope analyses (Geist et al., 2005) suggests a range of extended applications to assess the food quality and quantity requirements for juvenile and adult pearl mussels. Our understanding of adaptation and of the interactions between genotypes and environments can be improved by combining molecular genetic techniques with physiological and metabolic analyses (e.g. stable isotope methods) to investigate the functional link between genotypes and fitness parameters under different environmental conditions. These aspects will also be important for establishing sound breeding and culturing programmes for specific populations. Another main task will be to assess the long-term dynamics and viability of long-lived pearl mussel populations in correlation with their evolutionary potential, and to use ecological and genetic methods to understand the importance and interactions of multiple controlling factors with distribution and population structure of pearl mussels and their fish hosts. In particular, the influence of stream hydrological processes on microhabitat, particularly hydrodynamic effects on juvenile recruitment, is poorly understood (Skinner et al., 2003). Modelling the pathways of water runoff, nutrients and stressors in the catchments are important components for carrying out effective stream habitat restoration measures. Based on the current knowledge of the habitat requirements of pearl mussels, the reliability of practical conservation measures should be systematically tested. Restoration of functional stream beds has been shown to be successful (Altmüller & Dettmer, 2006) but needs to be modelled and tested under different environmental scenarios (e.g. flow regime, geology, geomorphology) to become more efficient.
On the species level, further survey work on the distribution and status of pearl mussel populations is needed. This especially should ensure making these data available for other researchers in the field. Currently, genetic analyses of samples from many geographical regions are being carried out. However, it is highly recommended that more populations representative of all different geographical regions are included into genetic investigations to study neutral divergence and adaptive variation of freshwater pearl mussels. Such studies in a more global context will help us to identify further priority populations for conservation and retain the maximum evolutionary potential. Genetic studies into Margaritifera margaritifera may additionally deliver important contributions to our knowledge about the historical, phylogenetic and phylogeographical processes of post-glacial colonisation patterns. In general, an improved data management on conservation actions, supportive breeding, stocking and mussel translocation will be mandatory for the systematic assessment of their effectiveness.
Above species level, one of the main tasks will be to gain a better understanding of the network of links between pearl mussels with their ecosystem and their importance for global biodiversity. This task includes further studies into co-occurrence patterns, the correlation of population fluctuations of pearl mussel and accessory species. C and N stable isotope analyses suggest investigations into the complex interactions of accessory species, food webs and the trophic-level organisation in functional and disturbed pearl mussel habitats. Owing to their comparatively sessile mode of life and longevity, pearl mussels and their distribution patterns can allow long-term interpretations of habitat factors and stream dynamics as well. Another interesting research approach will be to resolve the interrelation of patterns in the genetic structure between pearl mussels, their fish host vectors and other accessory species, and to assess these data in correspondence with differing life histories, demographic and stochastic effects. Studies of the genetic structure and biodiversity patterns of other freshwater bivalves with different modes of reproduction and in different habitat types can contribute to the understanding of the impacts of inbreeding depression under different reproductive strategies, and they can broaden the view of the genetic and ecological processes upon which mollusc biodiversity depends.
References
Altmüller, R. & R. Dettmer, 2006. Erfolgreiche Artenschutzmaßnahmen für die Flussperlmuschel Margaritifera margaritifera L. durch Reduzierung von unnatürlichen Feinsedimentfrachten in Fließgewässern – Erfahrungen im Rahmen des Lutterprojekts. Informationsdienst des Naturschutz Niedersachsen 26: 192–204.
Alvarez-Claudio, C., P. Garcia-Roves, R. Ocharan, J. A. Cabal, F. J. Ocharan & M. A. Alvarez, 2000. A new record of the freshwater pearl mussel Margaritifera margaritifera L. (Bivalvia: Unionoida) from the river Narcea (Asturias, north-western Spain). Aquatic Conservation: Marine and Freshwater Ecosystems 10: 93–102.
Araujo, R., Ramos, A., 2000. Action plan for Margaritifera margaritifera in Europe, Convention on the conservation of European Wildlife and natural habitats, Strasbourg: 41 pp. http://www.nature.coe.int/CP/tpvs10e.htm.
Araujo, R., Toledo, C., Van Damme, D., Ghamizi, M., Machordom, A., 2009. Margaritifera marocana (Pallary, 1918): a valid species inhabiting Moroccan rivers. Journal of Molluscan Studies, doi:10.1093/mollus/eyn043.
Barnhart, M. C., 2006. Buckets for muckets: a compact system for rearing juvenile freshwater mussels. Aquaculture 254: 227–233.
Bauer, G., 1986. The status of the freshwater pearl mussel in the south of its European range. Biological Conservation 37: 1–9.
Bauer, G., 1987a. Reproductive strategy of the freshwater pearl mussel Margaritifera margaritifera. Journal of Animal Ecology 56: 691–704.
Bauer, G., 1987b. The parasitic stage of the freshwater pearl mussel (Margaritifera margaritifera L.) III. Host relationships. Archiv für Hydrobiologie 76: 413–423.
Bauer, G., 1987c. The parasitic stage of the freshwater pearl mussel (Margaritifera margaritifera L.) II. Susceptibility of brown trout. Archiv für Hydrobiologie 76: 403–412.
Bauer, G., 1988. Threats to the freshwater pearl mussel Margaritifera margaritifera in central Europe. Biological Conservation 45: 239–253.
Bauer, G., 1992. Variation in the life span and size of the freshwater pearl mussel. Journal of Animal Ecology 61: 425–436.
Bauer, G., 1994. The adaptive value of offspring size among freshwater mussels (Bivalvia: Unionoidea). Journal of Animal Ecology 63: 933–944.
Bauer, G. & C. Vogel, 1987. The parasitic stage of the freshwater pearl mussel (Margaritifera margaritifera) I. Host response to glochidiosis. Archiv für Hydrobiologie 76: 393–402.
Bauer, G., S. Hochwald & W. Silkenat, 1991. Spatial distribution of freshwater mussels: the role of host fish an metabolic rate. Freshwater Biology 26: 377–386.
Bogan, A. E., 1993. Freshwater bivalve extinctions (Mollusca: Unionoida): a search for causes. American Zoologist 33: 599–609.
Bogan, A. E., 1998. Freshwater molluscan conservation in North America: problems and practises. Journal of Conchology Special Publication 2: 223–230.
Bogan, A., 2008. Global diversity of freshwater mussels (Mollusca, Bivalvia) in freshwater. Hydrobiologia 595: 139–147.
Bond, W. J., 1993. Keystone species. In Schulze, E. D. & H. A. Mooney (eds), Ecosystem Function and Biodiversity. Springer Verlag, Berlin: 237–253.
Boss, K. J., 1982. Mollusca. In Parker, S. P. (ed.), Synopsis and classification of living organisms, Vol. 1. McGraw-Hill, New York: 945–1116.
Bouchet, P., G. Falkner & M. B. Seddon, 1999. Lists of protected land and freshwater molluscs in the Bern Convention and European Habitats Directive: are they relevant to conservation. Biological Conservation 90: 21–31.
Bouza, C., J. Castro, P. Martínez, R. Amaro, C. Fernández, P. Ondina, A. Outeiro & E. San Miguel, 2007. Threatened freshwater pearl mussel Margaritifera margaritifera L. in NW Spain: low and very structured genetic variation in southern peripheral populations assessed using microsatellite markers. Conservation Genetics 8: 937–948.
Bowen, B. W., 1999. Preserving genes, species, or ecosystems? Healing the fractured foundations of conservation policy. Molecular Ecology 8: S5–S10.
Buddensiek, V., 1995. The culture of juvenile pearl mussels Margaritifera margaritifera in cages: a contribution to conservation programmes and the knowledge of habitat requirements. Biological Conservation 99: 183–190.
Buddensiek, V., H. Engel, S. Fleischauer-Rossing & K. Wächtler, 1993. Studies on the chemistry of interstitial water taken from defined horizons in the fine sediments of bivalve habitats in several North German lowland waters II: microhabitats of Margaritifera margaritifera L. Archiv für Hydrobiologie 127: 151–166.
Carell, B., S. Forberg, E. Grundelius, L. Henrikson, A. Johnels, U. Lindh, H. Mutvei, M. Olsson, K. Svardstrom & T. Westermark, 1987. Can mussel shells reveal environmental history? Ambio 16: 2–10.
Chesney, H. C. G. & P. G. Oliver, 1998. Conservation issues for Margaritiferidae in the British Isles and Western Europe. Journal of Conchology, Special Publication 2: 231–242.
Chesney, H. C. G., P. G. Oliver & G. M. Davis, 1993. Margaritifera durrovensis Phillips, 1928: taxonomic status, ecology and conservation. Journal of Conchology 34: 267–299.
Clark, J. A. & R. M. May, 2002. Taxonomic bias in conservation research. Science 297: 191–192.
Crandall, K. A., O. R. P. Bininda-Emonds, G. M. Mace & R. K. Wayne, 2000. Considering evolutionary processes in conservation biology. Trends in Ecology and Evolution 15: 290–295.
Cunjak, R. A. & S. E. McGladdery, 1991. The parasite-host relationship of glochidia (Mollusca: Margaritiferidae) on the gills of young-of-the-year Atlantic salmon (Salmo salar). Canadian Journal of Zoology 69: 353–358.
Davis, G. M. & S. L. H. Fuller, 1981. Genetic relationships among recent Unionacea (Bivalvia) of North America. Malacologia 20: 217–253.
Gaston, K. J. & R. M. May, 1992. The taxonomy of taxonomists. Nature 356: 281–282.
Geist, J., 1999a. Schadwirkungen von Feinsedimenten in Flussperlmuschelgewässern, die Flussmeister. Zeitschrift für Wasserwirtschaft, 43–46.
Geist, J., 1999b. Ist die Flussperlmuschel noch zu retten? Geoökologische Aspekte im Gewässerschutz. Junge wissenschaft 55: 18–24.
Geist, J., 2002. Entwicklung molekulargenetischer Marker bei der Flussperlmuschel (Margaritifera margaritifera L.). Diplomarbeit im Fach Biotechnologie der Tiere an der Technischen Universität München.
Geist, J. & K. Auerswald, 2007. Physicochemical stream bed characteristics and recruitment of the freshwater pearl mussel (Margaritifera margaritifera). Freshwater Biology 55: 2299–2316.
Geist, J. & R. Kuehn, 2005. Genetic diversity and differentiation of central European freshwater pearl mussel (Margaritifera margaritifera L.) populations: implications for conservation and management. Molecular Ecology 14: 239–425.
Geist, J. & R. Kuehn, 2008. Host-parasite interactions in pligotrophic stream ecosystems: the roles of life history strategy and ecological niche. Molecular Ecology 17: 997–1008.
Geist, J. & C. Schmidt, 2004. Besatzmaßnahmen mit Muscheln. Bayerns Fischerei und Gewässer No. 3/2004. I–IV.
Geist, J., O. Rottmann, W. Schröder & R. Kühn, 2003. Development of microsatellite markers for the endangered freshwater pearl mussel Margaritifera margaritifera L. (Bivalvia: Unionoidea). Molecular Ecology Notes 3: 444–446.
Geist, J., K. Auerswald & A. Boon, 2005. Stable carbon isotopes in freshwater mussel shells: environmental record or marker for metabolic activity? Geochimica et Cosmochimica Acta 69: 3545–3554.
Geist, J., M. Porkka & R. Kuehn, 2006. The status of host fish populations and fish species richness in European freshwater pearl mussel (Margaritifera margaritifera) streams. Aquatic Conservation: Marine and Freshwater Ecosystems 16: 251–266.
Geist, J., H. Wunderlich & R. Kuehn, 2008. Use of mollusc shells for DNA-based molecular analyses. Journal of Molluscan Studies 74: 337–343.
Geist, J., H. Söderberg, A. Karlberg & R. Kuehn, 2009. Drainage-independent genetic structure and high genetic diversity of endangered freshwater pearl mussel (Margaritifera margaritifera) in northern Europe. Conservation Genetics. doi: 10.1007/s10592-009-9963-4.
Haas, F., 1969a. Superfamilia Unionacea. In Das Tierreich, vol. 88 Walter de Gruyter and Co, Berlin.
Haas, F., 1969b. Superfamily Unionacea Fleming 1828. In: Moore, R. C. (ed.), Treatise of invertebrate paleontology, Part Mollusca 6, Vol 1. Bivalvia. Geological Society of America, Boulder, Colorado. University of Kansas press, Lawrence, Kansas: N411–N414.
Hastie, L. C. & P. J. Cosgrove, 2001. The decline of migratory salmonids: a new threat to pearl mussels in Scotland. Freshwater Forum 15: 85–96.
Hastie, L.C., Young, M.R., 2003a. Conservation of the freshwater pearl mussel 1. Captive breeding techniques. Conserving Natura 2000 Rivers Conservation Techniques Series No. 2. English Nature, Peterborough.
Hastie, L.C., Young, M.R., 2003b. Conservation of the freshwater pearl mussel 2. relationship with salmonids. Conserving Natura 2000 Rivers Conservation Techniques Series No. 3. English Nature, Peterborough: 44 pp.
Hastie, L. C., P. J. Boon & M. R. Young, 2000. Physical microhabitat requirements of freshwater pearl mussel, Margaritifera margaritifera (L.). Hydrobiologia 429: 59–71.
Heard, W. H. & R. H. Gluckert, 1970. A re-evaluation of the recent Unionacea (Pelecypoda) of North America. Malacologia 10: 333–335.
Hedrick, P. W., 2004. Recent developments in conservation genetics. Forest Ecology and Management 197: 3–19.
Henley, W. F., P. J. Grobler & R. J. Neves, 2006. Non-invasive method to obtain DNA from freshwater mussels (Bivalvia: Unionidae). Journal of Shellfish Research 25: 975–977.
Howard, J. K. & K. M. Cuffey, 2006. The functional role of native freshwater mussels in the fluvial benthic environment. Freshwater Biology 51: 460–474.
Huff, S. W., D. Campbell, D. L. Gustafson, C. Lydeard, C. Altaba & G. Giribet, 2004. Investigations into the phylogenetic relationships of freshwater pearl mussels (Bivalvia: Margaritiferidae) based on molecular data: implications for their taxonomy and biogeography. Journal of Molluscan Studies 70: 379–388.
Israel, W., 1913. Biologie der europäischen Süßwassermuscheln. K.G. Lutz Verlag, Stuttgart: 44–47.
Jansen, W., G. Bauer & E. Zahner-Meike, 2001. Glochidial mortality in freshwater mussels. In Bauer, G. & K. Wachtler (eds), Ecology and Evolutionary Biology of the Freshwater Mussels Unionoidea. Ecological Studies 145. Springer Verlag, Heidelberg: 185–211.
Johnson, R. I., 1970. Systematics and zoogeography of Plagiola (=Dysnomia = Epioblasma), an almost extinct genus of freshwater mussels (Bivalvia: Unionidae) from middle North America. Bulletin of the Museum of Comparative Zoology 148: 239–392.
Jones, J. W., E. M. Hallermann & R. J. Neves, 2006. Genetic management guidelines for captive propagation of freshwater mussels (Unionoidea). Journal of Shellfish Research 25: 527–535.
Jungbluth, J. R., Coomans, H. E., Groh, H., 1985. Bibliographie der Flussperlmuschel Margaritifera margaritifera (Linn. 1785), Verslagenen Technische Gegevens No 41, Institut voor Taxonomische Zoologie, Universiteit Amsterdam.
Larsen, B. M., 2001. Overvåking av elvemusling Margaritifera margaritifera i Norge, Årsrapport 2000. – NINA Oppdragsmelding 725: 1–43.
Lenormand, T., 2002. Gene flow and the limits to natural selection. Trends in Ecology & Evolution 17: 183–189.
Lindh, U., H. Mutvei, T. Sunde & T. Westermark, 1988. Environmental history told by mussel shells. Nuclear Instruments and Methods in Physics Research, Section B 30: 388–392.
Lydeard, C., Lindberg, D. R., 2003. In Lydeard, C., Lindberg D. R. (eds), Molecular Systematics and Phylogeography of Molluscs. Smithsonian Series in Comparative Evolutionary Biology. Smithonian Books Washington and London.
Lydeard, C., R. H. Cowie, W. F. Ponder, A. E. Bogan, P. Bouchet, A. S. Clark, K. S. Cummings, T. J. Frest, O. Gargominy, D. G. Herbert, R. Hershler, K. E. Perez, B. Roth, M. Seddon, E. E. Strong & F. G. Thompson, 2004. The global decline of nonmarine molluscs. Bioscience 54: 321–329.
Machordom, A., R. Araujo, D. Erpenbeck & M. A. Ramos, 2003. Phylogeography and conservation genetics of endangered European Margaritiferidae (Bivalvia: Unionoidea). Biological Journal of the Linnean Society 78: 235–252.
Manel, S., M. K. Schwartz, G. Luikart & P. Taberlet, 2003. Landscape genetics: combining landscape ecology and population genetics. Trends in Ecology and Evolution 18: 189–197.
Menge, B. A., E. L. Berlow, C. A. Blanchette, S. A. Navarette & S. B. Yamanda, 1994. The keystone species concept: variation in interaction strength in a rocky intertidal habitat. Ecological Monographs 64: 249–286.
Miguel, E. S., S. Monserrat, C. Fernández, R. Amaro, M. Hermida, P. Ondina & C. R. Altaba, 2004. Growth models and longevity of pearl mussels (Margaritifera margaritifera) in Spain. Canadian Journal of Zoology – Review Canadienne de Zoologie 82: 1370–1379.
Moorkens, E. A. & M. J. Costello, 1994. Imminent extenuation of the Nore freshwater pearl mussel Margaritifera durrovensis Phillips: a species unique to Ireland. Aquatic Conservation: Marine and Freshwater Ecosystems 4: 363–365.
Morales, J. J., A. I. Negro, M. Lizana, A. Martinez & J. Palacios, 2004. Preliminary study of the endangered populations of pearl mussels Margaritifera margaritifera (L.) in the river Tera (north-west Spain): habitat analysis and management considerations. Aquatic Conservation: Marine and Freshwater Ecosystems 14: 587–596.
Moritz, C., 1994. Defining evolutionary significant units for conservation. Trends in Ecology and Evolution 9: 373–375.
Moritz, C., 2002. Strategies to protect biological diversity and the evolutionary processes that sustain it. Systematic Biology 51: 238–254.
Moritz, C., Richardson, K. S., Ferrier, S., Monteith, G. B., Stanisic, J., Williams, S. E., Whiffin, T., 2001. Biogeographical concordance and efficiency of taxon indicators for establishing conservation priority in a tropical rainforest biota. Proceedings of the Royal Society of London B 268: 1875–1881.
Mutvei, H. & T. Westermark, 2001. How environmental information can be obtained from naiad shells. In Bauer, G. & K. Wächtler (eds), Ecology and Evolutionary Biology of the Freshwater Mussels Unionoidea. Ecological Studies 145. Springer Verlag, Heidelberg: 367–379.
Myers, N., 2003. Biodiversity hotspots revisited. BioScience 53: 916–917.
Nagel, K. O., G. Badino & G. Celebrano, 1998. Systematics of European Naiades (Bivalvia: Margaritiferidae and Unionidae): a review and some new aspects, Bivalvia I. Malacological Review 7: 83–104.
Neves, R. J., A. E. Bogan, J. D. Williams, S. A. Ahlstedt & P. W. Hartfield, 1997. Status of aquatic molluscs in the southeastern United States: a downward spiral of diversity. In Benz, G. W. & D. E. Collins (eds), Aquatic Fauna in Peril: The southeastern Perspective. Special Publication 1. Southeast Aquatic Research Institute, Lenz Design and Communications, Decatur, GA.
Nunney, L. & K. A. Campbell, 1993. Assessing minimum viable population size: demography meets population genetics. Trends in Ecology and Evolution 8: 234–239.
Outeiro, A., P. Ondina, C. Fernandez, R. Amaro & E. S. San Miguel, 2008. Population density and age structure of the freshwater pearl mussel, Margaritifera margaritifera, in two Iberian rivers. Freshwater Biology 53: 485–496.
Parodiz, J. J. & A. A. Bonetto, 1963. Taxonomy and zoogeographic relationships of the South American naiads (Pelecypoda: Unionacea and Mutelacea). Malacologia 1: 179–213.
Petit, R. J., E.l. Mousadik & O. Pons, 1998. Identifying populations for conservation on the basis of genetic markers. Conservation Biology 12: 844–855.
Ponder, W. F. & D. Lunney, 1999. The other 99%: The conservation and biodiversity of invertebrates. Royal Society of New South Wales, Mosman (Australia).
Preston, S. J., A. Keys & D. Roberts, 2007. Culturing freshwater pearl mussel Margaritifera margaritifera: a breakthrough in the conservation of an endangered species. Aquatic Conservation: Marine and Freshwater Ecosystems 17: 539–549.
Reed, D. H. & R. Frankham, 2003. Correlation between fitness and genetic diversity. Conservation Biology 17: 230–237.
Reis, J., 2003. The freshwater pearl mussel [Margaritifera margaritifera (L.)] (Bivalvia, Unionoida) rediscovered in Portugal and threats to its survival. Biological Conservation 114: 447–452.
Roe, K. J. & W. R. Hoeh, 2003. Systematics of freshwater mussels (Bivalvia: Unionoida). In Lydeard, C. & D. R. Lindberg (eds), Molecular Systematics and Phylogeography of Molluscs, Smithsonian Series in Comparative Evolutionary Biology. Smithonian Books, Washington and London: 91–122.
Ronce, O. & M. Kirkpatrick, 2001. The sources become sinks: migration meltdown in heterogenous habitats. Evolution: International Journal of Organic Evolution 55: 1520–1531.
Rudzite, M., 2004. Distribution of the freshwater pearl mussel Margaritifera margaritifera (Linnaeus 1758) in Latvia in relation to water quality. Acta Universitatis Latviensis, Biology 676: 79–85.
Sachteleben, J., Schmidt, C., Vandré, R., Wenz, G., 2004. Leitfaden Flussperlmuschelschutz. Bayerisches Landesamt für Umweltschutz (ed.), Augsburg.
Schmidt, C., Wenz, G., 2000. Kontinuierliche Überwachung der Flussperlmuschel in Bayern und Maßnahmen zur Bestandsstützung. In WWA Hof, Albert-Ludwigs-Universität Freiburg (eds), The Freshwater Pearl Mussel in Europe: Population Status and Conservation Strategies: 92–101.
Schmidt, C. & G. Wenz, 2001. Monitoring-Programm für ausgewählte Bestände der Flussperlmuschel (Margaritifera margaritifera L. 1758) als Datengrundlage für die Erfolgskontrolle von Schutzprojekten im Rahmen des Artenhilfsprogramms. BayLfU 156: 373–393.
Simberloff, D., 1998. Flagships, umbrellas, and keystones: is single-species management passé in the landscape era? Biological Conservation 83: 247–257.
Simberloff, D. & L. G. Abele, 1982. Refuge design and island biogeographic theory: effects of fragmentation. American Naturalist 120: 41–50.
Skinner, A., Young, M., Hastie, L., 2003. Ecology of the Freshwater Pearl Mussel. Conserving Natura 2000 Rivers Ecology Series No. 2 English Nature, Peterborough: 16 pp.
Smith, D. G., 2001. Systematics and distribution of the recent Margaritiferidae. In Bauer, G. & K. Wächtler (eds), Ecology and evolution of the freshwater mussels Unionoida. Springer Verlag, Heidelberg: 33–49.
Smith, D. G. & W. P. Wall, 1985. The Margaritiferidae reinstated: a reply to Davis and Fuller (1981) ‘Genetic relationships among recent Unionacea (Bivalvia) of North America’. Occasional Papers Molluscs 64: 321–332.
Soulé, M. E., 1987. Viable Populations for Conservation. Cambridge University Press, Cambridge.
Strayer, D., 2008. Freshwater Mussel Ecology: A Multifactor Approach to Distribution and Abundance. University of California Press, Berkeley, Los Angeles, London, ISBN 978-0-520-25526.
Strayer, D. L., J. A. Downing, W. R. Haag, T. L. King, J. B. Layzer, T. J. Newton & S. J. Nichols, 2004. Changing perspectives on pearly mussels, North America’s most imperiled animals. BioScience 54: 429–439.
Vaughn, C. C. & C. C. Hakenkamp, 2001. The functional role of burrowing bivalves in freshwater ecosystems. Freshwater Biology 46: 1431–1446.
Velasco Marcos, J. C., R. Araujo Armero, R. Bueno Hernandez & A. Laguna Gumiel, 2002. Descubierta la población europea más merional conocida de la madreperla de río Margaritifera margaritifera L. (Bivalvia, Unionoida), en la península Ibérica (Río Águeda, Salamanca). Discovered the southernmost known european population of the freshwater pearl mussel Margaritifera margaritifera L. (Bivalvia, Unionoida), in the Iberian peninsula (Río Águeda, Salamanca). Sociedad Espanola de Malacología. Iberus 20: 99–108.
Wächtler, K., M. C. Dreher-Mansur & T. Richter, 2001. Larval types and early postlarval biology in naiads (Unionoida). In Bauer, G. & K. Wächtler (eds), Ecology and Evolution of the Freshwater Mussels Unionoidea, Ecological Studies 145. Springer Verlag, Heidelberg: 93–125.
Watters, G. T., 1994. Form and function of unionoidean shell sculpture and shape (Bivalvia). American Malacological Bulletin 11: 1–20.
Watters, G. T., 2001. The evolution of the Unionacea in North America, and its implications for the worldwide fauna. In Bauer, G. & K. Wächtler (eds), Ecology and Evolution of the Freshwater Mussels Unionoidea. Ecological Studies 145. Springer Verlag, Heidelberg: 281–307.
Williams, J. D., M. L. Warren, K. S. Cummings, J. L. Harris & R. J. Neves, 1993. Conservation status of the freshwater mussels of the United States and Canada. Fisheries 18: 6–22.
Young, M. R. & J. C. Williams, 1983. The status and conservation of freshwater pearl mussel Margaritifera margaritifera L. in Great Britain. Biological Conservation 25: 35–52.
Young, M. R. & J. C. Williams, 1984. The reproductive biology of the freshwater pearl mussel Margaritifera margaritifera (Linn.) in Scotland. I. Field studies. Archiv für Hydrobiologie 99: 405–422.
Young, M. R., P. J. Cosgrove & L. C. Hastie, 2001. The extent of, and causes for, the decline of a highly threatened naiad: Margaritifera margaritifera. In Bauer, G. & K. Wächtler (eds), Ecology and Evolution of the Freshwater Mussels Unionoidea. Ecological Studies 145. Springer Verlag, Heidelberg: 337–357.
Young, M. R., L. C. Hastie & S. L. Cooksley, 2003. Monitoring the Freshwater pearl Mussel, Margaritifera margaritifera. Conserving Natura 2000 Rivers Monitoring Series No. 2. English Nature, Peterborough.
Ziuganov, V. V., Nezlin, L. P., 1988. Evolutionary aspects of symbiosis of pearl mussels and salmonid fishes. In The problems of macroevolution. Moscov. Nauka: 110–111.
Ziuganov, V., Zotin, A., Nezlin, L., Tretiakov, V., 1994. The freshwater pearl mussels and their relationships with salmonid fish. VNIRO, Russian Federal Institute of Fisheries and Oceanography, Moscow: 104 pp.
Ziuganov, V., S. Kaliuzhin, V. Beletsky & E. Popkovich, 2001. The pearl mussel-salmon community in the Varzuga river, northwest Russia: problems and environmental impacts. In Bauer, G. & K. Wächtler (eds), Ecology and Evolution of the Freshwater Mussels Unionoida. Ecological Studies 145. Springer Verlag, Heidelberg: 359–366.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: F. Thielen
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Geist, J. Strategies for the conservation of endangered freshwater pearl mussels (Margaritifera margaritifera L.): a synthesis of Conservation Genetics and Ecology. Hydrobiologia 644, 69–88 (2010). https://doi.org/10.1007/s10750-010-0190-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-010-0190-2