Abstract
We hypothesised that changes in nutritional composition and quality of the pelagic phytoplankton community influence the occurrence of Lesser Flamingo populations in two Kenyan saline–alkaline lakes, Nakuru and Bogoria. This was achieved by carrying out a detailed time series assessment of the phytoplankton community composition and nutritional components (carbohydrates, crude protein and lipids) from July 2008 to October 2009 on a weekly basis for each lake. Lesser Flamingos were estimated visually from the lake shore of the sampling sites. In Lake Nakuru, Lesser Flamingos had a significant positive relationship with lipids and Arthrospira biomass but a negative relationship with small cyanoprokaryotes. For Lake Bogoria, no significant differences were observed as the Arthrospira was available throughout the sampling period though the flamingo numbers still fluctuated. We concluded that the nutritional composition and quality of the phytoplankton community influence the temporal and spatial abundance of Lesser Flamingos although other factors such as the prevailing environmental conditions may take precedence.
Similar content being viewed by others
Introduction
In recent years, much work has been done to explain fluctuations and shifts in the phytoplankton community composition in the soda lakes of the East African Rift Valley and how they influence the abundances of Lesser Flamingos (Phoeniconaias minor Geoffrey) which inhabit these water bodies (Vareschi, 1978; Harper et al., 2003; Krienitz & Kotut, 2010). These saline–alkaline lakes are renowned for their abundant phytoplankton biomass dominated by the cyanobacterium Arthrospira fusiformis (Voronichin) Komárek & Lund (formerly called Spirulina plantensis Voronichin), which is the main food source for these birds (Vareschi, 1978; Owino et al., 2001). Besides having high pH, these lakes are also characterised by high temperatures, limited light penetration and high conductivity (Oduor & Schagerl, 2007). These factors create an unfavourable environment for most phytoplankton species other than the few well adapted cyanobacteria taxa. A. fusiformis, for example, dominates the phytoplankton biomass in these extreme biotopes over long periods where they almost form a unialgal population.
Lesser Flamingos exhibit unpredictable, spontaneous and sporadic nomadic movements between African alkaline–saline lakes that harbour their preferred food supply. They are able to migrate several hundreds of kilometres each day to access sites with suitable living conditions. It has been estimated that an adult Lesser Flamingo consumes up to about 72 g dry weight of cyanobacteria per day, mostly in shallow lake areas (Vareschi, 1978; Owino et al., 2001). This highly sought after food resource has been observed to undergo significant changes in its biomass over time. Ballot et al. (2004) observed changing dominance between A. fusiformis (hereinafter referred to as, Arthrospira) and Anabaenopsis abijatae Kebede & Willén in some saline lakes, which have been attributed to their response to the changing environmental stress expressed as fluctuations in physical and chemical variables (Schagerl & Oduor, 2008). Since Lesser Flamingos are specialised consumers of Arthrospira, such changes in biomass and quality are bound to affect the population of these birds in these soda lakes and in some cases, may also contribute to the sporadic mass mortalities observed at times.
Indeed, there have been reports of frequent unpredictable episodes of Lesser Flamingo mortalities in Kenyan Rift Valley lakes. In L. Nakuru, reports indicated sudden mortalities from 1993 to 2003, with deaths going beyond 500 in 1998 (Ndetei & Muhandiki, 2005). Krienitz & Kotut (2010) reported the highest mortality of flamingos (~30,000) in L. Nakuru in 2006. In 2008, the same authors also recorded about 30,000 carcasses of Lesser Flamingos that had accumulated along the shoreline of L. Bogoria. Among the possible causes for the sudden mortalities cited were the changes in food quantity (Sileo et al., 1979; Mlingwa & Baker, 2006) and composition (Ndetei & Muhandiki, 2005; Krienitz & Kotut, 2010). Problems with food composition are mainly associated with mucilage clogging the filters in the beaks and the production of cyanobacterial toxins by some taxa of cyanobacteria, which are ingested during feeding (Vareschi, 1978; Codd et al., 2003; Krienitz et al., 2003; Krienitz & Kotut, 2010).
Arthrospira is commonly regarded as non-toxic (Jassby, 1988) but some investigations have indicated possible toxicity of some of its strains (Gilroy et al., 2000; Iwasa et al., 2002). A toxic strain producing microcystin-YR and anatoxin-a was isolated from L. Sonachi, Kenya (Ballot et al., 2005). Microcystins (cyanobacterial hepatotoxins) and anatoxin-a (cyanobacterial neurotoxin) have been found to be present in cyanobacterial mats of the hot springs along the shores of L. Bogoria (Krienitz et al., 2003). These toxins also have been detected in the livers of dead Lesser Flamingos collected from L. Bogoria and L. Nakuru (Codd et al., 2003). The presence of hot spring cyanobacterial cells and cell fragments in stomach contents and faecal pellets show that flamingos ingest toxic cyanobacteria while drinking and washing at the hot springs (Krienitz et al., 2003). From these observations, cyanotoxins could be one factor contributing to the sudden deaths of the birds.
On the other hand, variations in nutritional quality, in terms of lipids, carbohydrates and crude protein contents of phytoplankton communities may also be another key factor contributing to the migration of these birds from one lake to the other, in search of phytoplankton with high nutritional value. Arthrospira (Spirulina spp.) nutrient components have been widely studied (Tokuşogulu & Ünal, 2003; Mühling et al., 2005; Zieliñska & Chojnacka, 2009). However, no studies so far have addressed variations in nutritional quality of phytoplankton community in terms of lipids, carbohydrates and crude protein content over short-term intervals in the natural environment. Krienitz & Kotut (2010) related flamingo movements to changes in algal food quantity and quality in terms of toxicity on an irregular basis. To our knowledge, a detailed time series assessment of the nutritional quality of the diet for the Lesser Flamingos on a regular basis has not previously been done in African saline lakes.
To address this gap, we carried out a detailed time series study of shifts in the pelagic phytoplankton community and the nutritional quality in terms of the crude protein, lipids and carbohydrates over short time intervals of 7 days and observed their effect on Lesser Flamingos in L. Nakuru and L. Bogoria. We hypothesised that changes in nutritional composition and quality of the pelagic phytoplankton community influence the occurrence of Lesser Flamingo populations.
Materials and methods
Study area and sites description
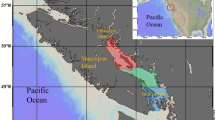
This study was carried out in two Kenyan Rift Valley lakes, L. Nakuru and L. Bogoria (Fig. 1), which are known to host huge flocks of Lesser Flamingos (Vareschi, 1978) with numbers sometimes going above 1 million birds between the lakes. L. Nakuru is a shallow pan situated in Lake Nakuru National Park, next to Nakuru Town. It is mainly recharged by rainfall. It also occasionally receives inflows from three seasonal surface streams, namely Njoro, Makalia and Nderit, which flow from the Mau Forest, and a small spring, the Baharini Spring at the northern end. Municipality sewage is also discharged into the lake. The lake hosts only one fish species (Oreochromis grahami alcalicus Linnaeus). The sampling point in L. Nakuru was in the central location at 00°21.387′S, 036°05.519′E. This point was chosen based on our preliminary observations which showed that it commonly hosts more Lesser Flamingos compared to the northern and southern parts of this lake. Also, due to the complete daily mixing of the lake, the data for physical and chemical variables collected from this point are representative of the whole lake (Oduor & Schagerl, 2007).
L. Bogoria lies in a semi-arid region in north-western Kenya. It is fed by few springs from the escarpment on the western side and some small impermanent tributaries that include the Emsoss on the eastern side and Wasagess River discharging from the northern side. The area around the lake is still volcanically active. Some boiling springs and fumaroles occur along the lake shore and discharge fresh to moderately alkaline–saline water into the lake. The lake receives erratic and stormy rainfall that erodes the scarcely vegetated areas around it, washing sediments into the lake. No macrophytes grow along its shoreline other than nearby the springs where some salt-tolerant grasses can be found (Harper et al., 2003). The lake does not support any fish life. The sampling point from this lake was at 00°16.166′N, 036°05.766′E. Similar to L. Nakuru, this sampling point was chosen as it is in the central area, which was observed to host higher flamingo numbers compared to the northern and southern parts of the lake.
Field sampling
Weekly sampling was carried out in each lake from July 2008 to October 2009. To capture seasonality, rainfall data was collected using HOBO RG3-M data loggers (Onset Computer Corporation, Massachusetts, USA), installed at weather stations located at the shores of both lakes. Salinity was measured at the sampling sites using a multiprobe (WTW Multi 340i Wissenschaftlich Technische Werkstätten Weilheim, Germany).
Flamingo numbers were estimated visually from the lake shore at least one sampling date per month at the sampling sites for both lakes from January to October 2009. The estimates were grouped into four size classes as follows: 1 = <10,000, 2 = 10,000 < 100,000, 3 = 100,000 ≤ 500,000 and 4 = >500,000.
Phytoplankton community composition and biomass determination
Phytoplankton community composition was determined using lake water samples fixed with formalin. The taxa were identified with the aid of established identification keys (Komárek, 2003; Komárek et al., 2003; Shubert, 2003; Kociolek & Spaulding, 2003). To determine the biovolume of the various community taxa, the identified cells for each taxa were then enumerated and measured using an inverted microscope (Nikon Diaphot, Nikon, Tokyo) at the ×100 and ×200 magnification (for Arthrospira and Anabaenopsis) and ×400 for other taxa according to Utermöhl (1958). Their biovolumes were estimated using geometric formulae of the shapes similar to the respective phytoplankton cells (Hillebrand et al., 1999). At least 30 cells for each taxon identified were measured to give the average size and biovolume. For conversion of cell volume into biomass, a conversion factor of 1 was used (Wetzel & Likens, 1991).
Dry and ash mass, carbohydrate, lipid and crude protein determination
Dry mass and ash mass was analysed by filtering a known volume of the raw sample using pre-weighed filters (45-μm pore size; Ederol BM/C, Battenburg, Germany). For dry mass, filters together with residue material were dried at 95 ± 5°C until a constant weight was achieved. For estimation of the ash mass, dry mass filters were combusted in a muffle furnace for 2 h at 550°C. The weight loss on ignition gave the ash-free dry mass (organic content).
A plankton net of 30 μm was used to collect samples so as to harvest algal particles that can go through the excluders of the Lesser Flamingo bill and are retained and secondly to obtain adequate material for nutritional components analysis. These samples were analysed for carbohydrates, crude protein and lipids which were extracted according to the methods described by Schwörbel (1994) with appropriate modifications. Net samples were then filtered through filter papers with mesh size 0.45 μm (Ederol BM/C, Battenburg, Germany) and the residue together with the filter ground using a tissue grinder. Where immediate analysis was not possible, the samples were kept frozen at −20°C.
Carbohydrates were determined using the phenol–sulphuric acid complex reaction; controls were prepared in a similar way, using distilled water instead of the net sample, and β-d-glucose was used for preparation of the standards. The vanillin reaction method was used to determine the sample lipid concentration. Controls were prepared in a similar way using distilled water instead of raw material from the samples. A calibration factor of 1,805 for algae was used (Schwörbel, 1994). Crude protein was measured following the Biuret method as described by Schwörbel (1994) with controls prepared with distilled water; bovine serum albumin was used for calibration.
Statistics
For group comparisons, flamingo numbers were classified within each lake into four categories (ref. field sampling) and tested with Kruskal–Wallis statistics for significant differences of potential explanatory variables like food quality in terms of lipids, carbohydrates, proteins and occurrence of Arthrospira and other algal groups. Calculations were done with the software package SPSS 20.0.
Results
Temporal changes in phytoplankton biomass were observed in both lakes as illustrated in Fig. 2. Biomass fluctuation was greater in L. Nakuru compared to L. Bogoria. The lowest phytoplankton biomass in L. Nakuru was recorded in December 2008 as 6.4 mg l−1 while the highest recorded value of 248.4 mg l−1 was in June 2009. Over time, the contribution of Arthrospira to the total phytoplankton biomass in L. Nakuru was quite variable (Fig. 3). Most of the time, Arthrospira contributed over 80% of the total biomass during the wet seasons that occurred between August and November 2008 and April to August 2009, with correspondingly low salinity levels (16–20 ppt) in the lake.
A notable phenomenon in L. Nakuru was the crash in the biomass of Arthrospira that occurred from January to April, 2009 and September to October, 2009 (Fig. 3), coinciding with high salinity levels (62 ppt) when rainfall was low (Fig. 2). During these dry periods, other phytoplankton taxa, in particular, A. abijatae, cryptomonads and other cyanobacteria (Synechoccus minutus West, Synechocystis sp., Raphidiopsis sp.) dominated the biomass of the phytoplankton community in the lake (Fig. 3). In addition, in October 2009, a bloom of an unidentified filamentous cyanobacterium species (cf. Pseudanabaena acicularis (Nygaard) Anagnostidis et Komárek) emerged, forming a thick mat on the lake surface. Green algae (Ankistrodesmus sp., Crucigenia sp. and Monoraphidium minutum (Nägeli) Kormárková-Legnerová), diatoms (Nitzschia and Navicula) and euglenoids (Euglena sp.) contributed 6% to the phytoplankton biomass.
Carbohydrate and lipid composition of the organic matter in L. Nakuru ranged from 2.6–76.3 to 0.6–42.4%, respectively, whereas crude protein ranged from 18.8 to 94.5% (Fig. 4). The lowest carbohydrate and lipid content (2.6 and 0.6%, respectively) occurred in December 2008 and February 2009, coinciding with the first Arthrospira biomass crash (0.4–2.9 mg l−1). Crude protein composition (Fig. 4) was generally high between November 2008 and March 2009 (70–95%) when the salinity was low (16–28 ppt) but concentrations decreased, reaching 20% from March to October 2009 as salinity levels increased steadily to 62 ppt, which coincided with the period of low rainfall (Fig. 2). On the other hand, it was observed that there was an increase in carbohydrate and lipid composition, with carbohydrate exceeding crude protein composition in October 2009 (Fig. 4). Dry mass concentrations in Lake Nakuru fluctuated between 0.04 and 1.43 g l−1 (Fig. 4). The minimum value occurred in January 2009 during the period of the first Arthrospira crash, while the maximum occurred in October 2009. For the greater part of the sampling period, the ratio of ash mass to ash-free dry mass was 1:1 but the pattern changed in September 2009 to 2:1, respectively.
Generally, there was an inverse relationship between Lesser Flamingo populations in L. Nakuru and L. Bogoria for the greater part of sampling period (Fig. 5). Lesser Flamingo numbers in L. Nakuru showed temporal fluctuation that mirrored the changes in Arthrospira biomass (Fig. 3). There were no more than 100,000 flamingos during the period of January to March 2009, coinciding with the first Arthrospira biomass crash observed during this study. In April 2009, flamingo numbers started to rise, increasing further from June to August to over 500,000, and matching with the increase in Arthrospira biomass during the same period. The numbers dropped again in September and October 2009 to less than 10,000, corresponding to the second Arthrospira biomass crash and a very low organic matter content (Fig. 4). During this time, Arthrospira was replaced by a mono-specific dominance of a filamentous cyanobacterium (cf. P. acicularis). It is also worth noting that no flamingo deaths were observed in L. Nakuru during this study. Kruskal–Wallis test indicated significant differences (P < 0.01) among the four categories of Lesser Flamingo estimates in relation to lipids, Arthrospira biomass and biomass of “other cyanoprokaryotes”, which subsume all cyanopokaryotes except Arthrospira and Anabaenopsis (Fig. 6).
L. Bogoria received a total of 353 mm of rainfall from July to December 2008 with a brief dry period from September to October, 2008. The marked temporal fluctuations in salinity levels and total phytoplankton biomass that were observed in L. Nakuru were not very evident in L. Bogoria even though rainfall was quite variable throughout the study. As reported for L. Nakuru, a distinct dry period was also evident from January to March 2009, followed by increase in rainfall from April to July 2009 and a brief dry period again from August to September 2009 in L. Bogoria. The salinity range in L. Bogoria was narrow, 37–41 ppt from July to December 2008, slightly increasing with the onset of the dry period to 49 ppt towards October 2009 (Fig. 2).
Phytoplankton biomass in L. Bogoria ranged from a minimum of 21.4 mg l−1 in October 2009 to a maximum of 153.5 mg l−1 recorded in June 2009 (Fig. 2). As in L. Nakuru, Arthrosipra contributed the largest proportion of biomass to the total phytoplankton biomass in L. Bogoria, averaging 92 ± 1.1 (SE)% (Fig. 3). Unlike L. Nakuru, no big variation was observed in the phytoplankton composition in L. Bogoria. Arthrospira exhibited a more or less mono-specific dominance in this lake, contributing more than 80% of the total phytoplankton biomass throughout the study. Other phytoplankton taxa that occurred in decreasing order of importance included cryptomonads, green coccal algae like Picocystis salinarum Lewin, M. minutum) and diatoms (Nitzschia sp.), which in total contributed about 8% to the total phytoplankton biomass (Fig. 3).
Carbohydrate content of organic matter in L. Bogoria varied between 8 and 70% whereas lipids ranged from 3 to 37% (Fig. 7). The variations in carbohydrate and lipid content in L. Bogoria did not follow any distinct seasonal pattern nor did they reflect the temporal Arthrospira biomass. Generally, crude protein content was higher than carbohydrate or lipid contents with values up to 88%. There was no marked variation in crude protein content even with the changes in the seasonal rainfall. Dry mass concentrations ranged from 0.1 (March 2009) and 0.4 g l−1 (July 2008) throughout the sampling period (Fig. 7). Ash mass and ash-free dry mass concentrations occurred in a ratio of ~1:1 for the whole sampling period.
Compared to L. Nakuru, changes in Lesser Flamingo numbers in L. Bogoria seemed unresponsive to Arthrospira biomass in the lake (Fig. 3). For L. Bogoria, there were no observed significant differences among the flamingo categories and the nutritional quality components, Arthrospira biomass and other algae.
There were about 100,000 Lesser Flamingos in L. Bogoria in January and February 2009, with many juveniles observed in February as Arthrospira biomass levels remained below 100 mg l−1. The number of Lesser Flamingos increased in March to about 500,000. From April to July 2009, Lesser Flamingo numbers ranged between 10,000 and 500,000 even though Arthrospira biomass increased beyond 100 mg l−1. High numbers of juvenile Lesser Flamingos were observed again in June while in July, it was observed that the birds were scattered all over the lake, with Arthrospira biomass remaining relatively high. From August to October 2009, the lake hosted the highest number of Lesser Flamingos, with numbers above 500,000. Though no Lesser Flamingo counts were done for the period of July to December 2008, it is worthwhile to mention that in July 2008, 50,000 flamingo carcasses were observed along the shores of L. Bogoria. In addition, about 150 and 100 flamingo carcasses were seen in March and August 2009, respectively, along the lake shore.
Discussion
In spite of the high temporal variations observed for phytoplankton biomass in L. Nakuru, with peaks sometimes surpassing those observed for L. Bogoria, a higher and more stable phytoplankton biomass occurred in L. Bogoria compared to L. Nakuru. This was as a result of the physical and chemical stability of L. Bogoria as similarly observed by other researchers (Harper et al., 2003). Alternatively, the high fluctuations of environmental conditions in L. Nakuru hamper stable growth of phytoplankton. The sensitivity of L. Nakuru to seasonal weather changes is due to the morphological nature of L. Nakuru as a shallow pan exposes more water to evaporation that results into a more rapid water loss as compared to L. Bogoria. Shallow lakes are also known for high loading of suspended organic materials as a result of river inflow, sediment disturbance by wading birds or through wind action (Ballot et al., 2004). Secondly, the wind induced patchiness, which is common with scum forming algae, especially in shallow lakes (Blukacz et al., 2009).
Being the main diet of Lesser Flamingos (Owino et al., 2001), these birds tend to migrate in response to changes in Arthrospira biomass in these lakes (Nasirwa, 2000). Arthrospira is known to have an exceptionally high protein content of about 65% of its dry mass, while total lipids and carbohydrates constitute between 7 and 15%, respectively (Tokuşoglu & Ünal, 2003), which overall results into a relatively low caloric equivalent of 4,360 cal g−1 DM (Vareschi, 1978). This nutritional composition was also reflected in our food analyses for both L. Nakuru and L. Bogoria.
In L. Nakuru, the Lesser Flamingos thrived best during the period of the highest Arthrospira biomass, which prevailed during suitable environmental conditions in the rainy season offering more fresh water and a cooler environment. However, with the increase in flamingo numbers the grazing pressure on the Arthrospira increased resulting into a reduction in biomass and consequently a decline in Lesser Flamingos numbers. When Arthrospira biomass falls below a certain threshold, Lesser Flamingos are not able to obtain enough food to meet their energy requirements. As a result, they move to other lakes with a more favourable food base or at the worst, they are forced to change diets to the less desirable phytoplankton taxa (Sileo et al., 1979; Tuite, 2000). We opine that this may have been the reason for the flamingo fluctuations between the two lakes as the birds tend to favour the lake with better living conditions at a given time.
For example, if the food supply becomes a limiting factor in L. Nakuru, the flamingos have to find other sources even moving towards the harsh environment of L. Bogoria leading to interlake movements as also observed by Chidress et al. (2004) and Tuite (1979). Lakes Nakuru and Bogoria are part of the key site network consisting of eight alkaline lakes—Logipi, Elmenteita, Natron, Empakayi Crater Lake, Manyara and Eyasi, which are the main home to the Lesser Flamingos (Childress et al., 2007) in the Eastern Rift Valley.
During the period of Arthrospira crash, Anabaenopsis, other cyanobacteria and cryptomonads dominated. These phytoplankton taxa are less preferred by the Lesser Flamingos, consequently the presence of very low flamingo numbers that occurred at this time. For instance, the large slimy lumps of Anabaenopsis may clog the excluders and lamellae of flamingos hence preventing food uptake, which could lead to starvation and consequently, malnutrition (Krienitz & Kotut, 2010). Moreover, single-celled cyanoprokaryotes, cryptomonads and diatoms are much too small to be held back effectively.
Increased grazing pressure on Arthrospira, affected the nutritional quality as well, as was shown by a similar reduction in the amount of lipids. Our results for carbohydrates and crude protein did not show any significant relationship with the Lesser Flamingo categories as expected, possibly due to interference from other food sources since the method of sampling used was non-selective thereby including other organic material. Vareschi (1978) observed that dense rotifer populations can substantially contribute to the Lesser Flamingo diet in times of very low Arthrospira densities.
In L. Bogoria, it is more likely that environmental conditions may have taken precedence over the nutritional composition and quality in regulating the abundance of Lesser Flamingos at this lake. For instance, the extremely hot weather conditions could have been stressful for the flamingos hence the fluctuation in numbers even when there was adequate Arthrospira biomass.
The temporal pattern observed of higher crude protein composition during the wet period in L. Nakuru compared to the dry period was most probably as a result of the changes in salinity levels that occurred at that time. During the wet period, the lowered salinity levels may have been favourable for higher protein production by the phytoplankton and therefore offering a more nutritious diet to the Lesser Flamingos. This was supported by the exceptionally high numbers that prevailed during this period. Vonshak & Tomaselli (2000) have shown that at high salinities, protein levels in Arthrospira decrease while carbohydrates increase as an adaptation for efficient osmoregulation. In addition from the experiments conducted by Apte & Bhagwat (1989) on two Anabaena strains, they observed that exposure to high salinity resulted in inhibition of protein synthesis.
The high flamingo mortalities that were observed in July 2008 at L. Bogoria were comparable to those observed by Krienitz and Kotut (2010) who recorded about 30,000 flamingo carcasses. Although comparably fewer flamingo deaths occurred in March and August 2009, we noted that both mortalities occurred when the highest flamingo numbers were recorded at this lake. Arthrospira contributed above 80% of the total phytoplankton biomass in the lake which indicated that the food quantity was sufficient. Given that Arthrospira dominance remained unchanged in L. Bogoria and the nutritional quality, as reflected in carbohydrates, lipids and crude protein was also relatively stable, the observed flamingo fluctuations and mortalities at the lake could not be directly associated with low food availability nor could they be due to poor nutritional quality.
One possible food related cause for the observed mortalities could be that the filtration system of the juvenile Lesser Flamingos may have been clogged due to the high phytoplankton biomass resulting into death. Vareschi (1978) observed that Lesser Flamingos avoid areas of aggregated algae, which is a common occurrence in L. Bogoria, possibly because their filter system would become clogged. It is also thought that mortality in juvenile birds is higher than for adults (Vareschi, 1978). On several occasions in L. Bogoria, we observed especially in the afternoons, strong winds blow the phytoplankton towards the shores leading to formation of thick algal mats. The fact that there were many juveniles in L. Bogoria at this time suggests that probably they had just arrived from their breeding ground at L. Natron. The breeding season for Lesser Flamingos is known to take place between October and January (Childress et al., 2007).
Ingestion of cyanobacterial toxins by the Lesser Flamingos during feeding has also been cited by Codd et al. (2003) and Krienitz et al. (2003) as another possible cause of Lesser Flamingo mortalities in these lakes. For this study, we did not find cyanotoxins cyanobacteria (unpublished results) so we can exclude toxins as a key factor for flamingo deaths during our study period.
We agreed with Krienitz & Kotut (2010), that the problems of phytoplankton food quantity and quality facing the Lesser Flamingos are indeed still quite complex and there is still much more to be discovered. Therefore, we concluded that the nutritional composition and quality of the phytoplankton community influence the temporal and spatial abundance of Lesser Flamingos in these soda lakes although other factors such as the prevailing environmental conditions may take precedence over nutritional composition and quality.
References
Apte, K. S. & A. Bhagwat, 1989. Salinity-stress-induced proteins in two nitrogen-fixing Anabaena strains differentially tolerant to salt. Journal of Bacteriology 171: 909–915.
Ballot, A., L. Krienitz, K. Kotut, C. Wiegand, J. S. Metcalf, G. A. Codd & S. Pflugmacher, 2004. Cyanobacteria and cyanobacterial toxins in three alkaline rift valley lakes of Kenya—Lakes Bogoria, Nakuru and Elmenteita. Journal of Plankton Research 26: 925–935.
Ballot, A., L. Krienitz, K. Kotut, C. Wiegand & S. Pflugmacher, 2005. Cyanobacteria and cyanobacterial toxins in alkaline crater lakes Sonachi and Simbi, Kenya. Harmful Algae 4: 139–150.
Blukacz, E. A., B. J. Shutter & W. G. Sprules, 2009. Towards understanding the relationship between wind conditions and plankton patchiness. Limnology and Oceanography 54: 1530–1540.
Childress, B., D. Harper, B. Hughes, W. van den Bossche, P. Berthold & U. Querner, 2004. Satellite tracking Lesser Flamingo movements in the Rift Valley, East Africa: pilot study. Ostrich 75: 57–65.
Childress, B., B. Hughes, D. Harper & W. van den Bossche, 2007. East African flyway and key site network of the Lesser Flamingo (Phoenicopterus minor) documented through satellite tracking. Ostrich 78: 463–468.
Codd, A. C., J. S. Metcalf, L. F. Morrison, L. Krienitz, A. Ballot, S. Pflugmacher, C. Wiegand & K. Kotut, 2003. Susceptibility of flamingos to cyanobacterial toxins via feeding. The Veterinary Record 152: 722–723.
Gilroy, D. J., K. W. Kauffman, R. A. Hall, X. Huang & F. S. Chu, 2000. Assessing potential health risks from microcystin toxins in blue-green algae dietary supplements. Environmental Health Perspectives 108: 435–439.
Harper, D. M., R. B. Childress, M. M. Harper, R. R. Boar, P. Hickley, S. C. Mills, N. Otieno, T. Drane, E. Vareschi, O. Nasirwa, W. E. Mwatha, J. P. E. C. Darlington & X. Escute-Gasulla, 2003. Aquatic biodiversity and saline lakes: Lake Bogoria National Reserve, Kenya. Hydrobiologia 500: 259–276.
Hillebrand, H., C. D. Durselen, D. Kirschtel, U. Pollingher & T. Zohary, 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology 35: 403–424.
Iwasa, M., M. Yamamoto, Y. Tanaka, M. Kaito & Y. Adachi, 2002. Spirulina-associated hepatotoxicity. American Journal of Gastroenterology 97: 3212–3213.
Jassby, A., 1988. Spirulina: a model for microalgae as human food. In Lembi, C. A. & J. R. Waaland (eds), Algae and Human Affairs. Cambridge University Press, Cambridge: 149–179.
Kociolek, P. J. & S. A. Spaulding, 2003. Symmetrical naviculoid diatoms. In Wehr, D. J. & R. G. Sheath (eds), Freshwater Algae of North America: Ecology and Classification. Academic Press, San Diego: 637–639.
Komárek, J., 2003. Coccoid and colonial cyanobacteria. In Wehr, D. J. & R. G. Sheath (eds), Freshwater Algae of North America: Ecology and Classification. Academic Press, San Diego: 59–110.
Komárek, J., H. Kling & J. Komárková, 2003. Filamentous cyanobacteria. In Wehr, D. J. & R. G. Sheath (eds), Freshwater Algae of North America: Ecology and Classification. Academic Press, San Diego: 117–191.
Krienitz, L. & K. Kotut, 2010. Fluctuating algal food populations and the occurrence of Lesser Flamingos (Phoeniconaias minor) in three Kenyan Rift Valley lakes. Journal of Phycology 46: 1088–1096.
Krienitz, L., A. Ballot, K. Kotut, C. Wiegand, S. Putz, J. S. Metcalf, G. A. Codd & S. Pflugmacher, 2003. Contribution of hot spring cyanobacteria to the mysterious deaths of Lesser Flamingos at Lake Bogoria, Kenya. Fems Microbiology Ecology 43: 141–148.
Mlingwa, C. & N. Baker, 2006. Lesser Flamingo Phoenicopterus minor counts in Tanzanian soda lakes: implications for conservation. In Boere, G. C., C. A. Galbraith & D. A. Stroud (eds), Waterbirds Around the World. The Stationery Office, Edinburgh: 230–233.
Mühling, M., A. Belay & B. A. Whitton, 2005. Variation in fatty acid composition of Arthrospira (Spirulina) strains. Journal of Applied Phycology 17: 137–146.
Nasirwa, O., 2000. Conservation status of Flamingos in Kenya. Waterbirds 23: 47–51.
Ndetei, R. & V. S. Muhandiki, 2005. Mortalities of lesser flamingos in Kenyan Rift Valley saline lakes and the implications for sustainable management of the lakes. Lakes & Reservoirs Research and Management 10: 51–58.
Oduor, S. O. & M. Schagerl, 2007. Phytoplankton primary productivity characteristics in response to photosynthetically active radiation in three Kenyan Rift Valley alkaline-saline lakes. Journal of Plankton Research 29: 1041–1050.
Owino, A. O., J. O. Oyugi, O. O. Nasirwa & L. A. Bennun, 2001. Patterns of variation in waterbird numbers on four Rift Valley lakes in Kenya, 1991–1999. Hydrobiologia 458: 45–53.
Schagerl, M. & S. O. Oduor, 2008. Phytoplankton community relationship to environmental variables in three Kenyan Rift Valley saline–alkaline lakes. Marine and Freshwater Research 59: 125–136.
Schwörbel, J. 1994. Methoden der Hydrobiologie, 4 edn. Gustav Fischer Verlag, Stuttgart, Jena: 81, 238.
Shubert, E., 2003. Nonmotile coccoid and colonial green algae. In Wehr, D. J. & R. G. Sheath (eds), Freshwater Algae of North America: Ecology and Classification. Academic Press, San Diego: 253–308.
Sileo, L., J. G. Grootenhuis, C. H. Tuite & J. B. D. Hopcraft, 1979. Mycobacteriosis in the Lesser Flamingos of Lake Nakuru, Kenya. Journal of Wildlife Diseases 15: 387–389.
Tokuşoglu, Ö. & M. K. Ünal, 2003. Biomass nutrient profiles of microalgae: Spirulina platensis, Chlorella vulgaris and Isochrisis galbana. Food Chemistry and Toxicology 68: 4.
Tuite, C. H., 1979. Population size, distribution and biomass density of the Lesser Flamingo in the Eastern Rift Valley, 1974–76. Journal of Applied Ecology 16: 765–775.
Tuite, C. H., 2000. The distribution and density of Lesser Flamingos in East Africa in relation to food availability and productivity. Waterbirds 23: 52–63.
Utermöhl, H., 1958. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitteilungen der Internationalen Vereinigung für Theoretische und Angewandte Limnologie 9: 1–38.
Vareschi, E., 1978. Ecology of Lake Nakuru (Kenya). I. Abundance and feeding of Lesser Flamingo. Oecologia 32: 11–35.
Vonshak, A. & L. Tomaselli, 2000. Arthrospira (Spirulina): systematics and ecophysiology. In Whitton, A. B. & M. Potts (eds), The Ecology of Cyanobacteria. Their Diversity in Time and Space. Kluwer Academic Publishers, The Netherlands: 505–522.
Wetzel, R. G. & G. E. Likens, 1991. Limnological Analyses, 2nd ed. Springer Verlag, New York.
Zieliñska, A. & K. Chojnacka, 2009. The comparison of biosorption of nutritionally significant minerals in single- and multi-mineral systems by the edible microalga Spirulina sp. Journal of the Science of Food and Agriculture 89: 2292–2301.
Acknowledgments
The authors wish to thank the Kenya Government for granting them a research permit to carry out research in the two lakes. They also thank the Kenya Wildlife Services (KWS) and the Lake Bogoria Game Reserve authorities for granting them access to Lake Nakuru National Park and Lake Bogoria Game Reserve, respectively. We also acknowledge and appreciate the financial assistance offered by the Austrian Partnership Programme in Higher Education and Research for Development (APPEAR). Many thanks as well, to Pauline Macharia and Bernerd Simuyu who assisted during the sampling and laboratory analyses. This study was funded by the Austrian Science Fund Project No. P19911 “Factors controlling abundance of Arthrospira fusiformis”.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Zhengwen Liu, Bo-Ping Han & Ramesh D. Gulati / Conservation, management and restoration of shallow lake ecosystems facing multiple stressors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kaggwa, M.N., Gruber, M., Oduor, S.O. et al. A detailed time series assessment of the diet of Lesser Flamingos: further explanation for their itinerant behaviour. Hydrobiologia 710, 83–93 (2013). https://doi.org/10.1007/s10750-012-1105-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1105-1