Abstract
Pike in the western Baltic Sea live on the edge of their salinity tolerance. Under physiologically challenging conditions, organism may respond by moving to environmentally more benign areas during critical periods, such as during spawning. We hypothesised that pike in a brackish lagoon (8–10 ppt salinity) would perform spawning- and feeding-related movements between areas with different salinity regimes. Twenty-two pike were caught prior to spawning, tagged with acoustic transmitters, and their movements were tracked for 18 months. Pike showed two main patterns of movements that were consistent within individuals across two years. Whereas some individuals stayed in the lagoon year-round, most pike left the lagoon for longer periods after spawning and returned to the lagoon prior to following year’s spawning season. We found no evidence that probability of moving out of the lagoon co-varied with either length or condition factor. Despite the fact that the lagoon’s salinity is close to the reported upper limit for pike egg development, results indicated that all pike spawned in the lagoon. Correspondingly, genetic data showed that all fish belonged to the same reproductive population unit. Movement patterns thus appear to reflect individual variation in home-range and/or resource optimisation following ideal free principles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Baltic Sea is one of the world’s largest brackish water bodies. Yet, there is limited knowledge about the biology and behaviour of several of the freshwater fish species living in this area on the edge of their upper salinity tolerances. This is particularly true for populations inhabiting the transition zone between brackish water and marine salinities constituting a physiologically challenging environment for the organisms living here. The western Baltic Sea around the south-eastern part of the Danish straits is such a transition zone. Here, water from the Baltic Sea of 6–7 ppt meets water from the North Sea entering through the Kattegat (c. 20 ppt). Around the south-eastern islands of Denmark mean salinity is around 8–12 ppt. The hydrological conditions are complex and the salinity varies greatly with higher concentrations when strong winds from westerly direction at irregular intervals force high-salinity water into the area. High-salinity water can be trapped during such periods around smaller islands and bays, whereas other areas with freshwater outlets may maintain lower levels of salinity. Conversely, lower salinity is encountered in the area when specific wind directions force brackish water out of the Baltic (Fischer & Matthäus, 1996).
In spite of being a freshwater species, northern pike (Esox lucius L.) is widely distributed throughout coastal areas in the Baltic. Studies on behaviour and migration in the eastern areas, where salinity normally is 6–7 ppt, show that some pike spawn at sea, whereas others exhibit annual spawning migrations to rivers and lakes (Müller, 1986; Karås & Lehtonen, 1993; Westin & Limburg, 2002; Engstedt, 2011; Rohtla et al., 2012). The advantage of freshwater spawning migration is hypothesised to be access to higher temperatures and more protected, vegetated spawning areas, with ample spawning substrate, cover and food for larvae (Karås & Lethonen, 1993; Engstedt, 2011).
Pike is also found in coastal waters of the south-western part of the Baltic, where salinities are 10–12 ppt (temporarily up to 14–15 ppt). The physiological tolerance to salinity is not well understood in pike, but mass mortality of pike was reported from brackish areas in periods with incidents of salinities of more than 18 ppt (Dahl, 1961). Fertilisation and egg development was successful at 8.5 ppt under experimental conditions, and pike fry exhibited normal behaviour in brackish water less than 13.5 ppt in a population from the W Baltic transition zone (Jørgensen et al., 2010). However, the behaviour of pike living near their upper salinity tolerance is poorly described. It is unknown whether pike under such conditions perform spawning migration to freshwater locations, or are able to complete their life cycle in areas with such high salinity. We describe the annual movements of individual pike inhabiting a brackish lagoon of 8–10 ppt by use of acoustic telemetry. We hypothesised that all pike or part of the population would perform movements related to spawning or feeding between areas with different salinity regimes, and in particular we expected tagged pike to enter freshwater outlets during the spawning period.
Materials and methods
Study site
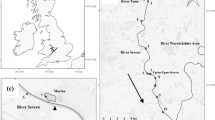
The study was carried out in Stege Lagoon (54°58′45N, 12°17′47E), situated on the island of Møn southeast of Zealand, Denmark. The lagoon covers 5.69 km2 and is shallow with a mean depth of 1.3 m (maximum depth 3.7 m). It is connected to Stege Bay through a narrow outlet (width c. 60 m). There is only one minor freshwater inlet in the north-eastern part, Lendemark Brook (Fig. 1). Salinity in the Lagoon is normally 8–10 ppt, but can be higher during periods with inflowing saline waters (see Fig. 2a). The lagoon has shallow areas with vegetation, mainly bladder weed (Fucus vesiculosus L.) and Potamogeton pectinatus L. along with reed belts (Phragmites australis (Cav.)). The fish population in the lagoon included pike, perch (Perca fluviatilis L.), roach (Rutilus rutilus (L.)), eel (Anguilla anguilla L.), black goby (Gobius niger L.), stickleback (Gasterosteus aculeatus L.) and sea stickleback (Spinachia spinachia (L.)) (L. Jacobsen, unpublished data).
a Salinity (black line) and water temperature (dotted line) in Stege Lagoon during the study period (grey bar) including a period before and after the study period (September 2002–May 2005). Both parameters were measured in the harbour area. b Salinity at three locations in the lagoon: harbour area (receiver #8, stippled line), north-eastern end (receiver #1, open square) and the southern part (receiver # 6, grey triangle). The three locations represent the salinity extremes in the lagoon
Material
In March 2003, 22 pike were caught by angling from boats using artificial lures. Pike were kept overnight in a wooden livewell. Before tagging, pike were anaesthetised with benzocaine and placed on a surgical pillow. An acoustic coded transmitter (V13-1L, weight in water: 6 g, random code burst interval: 40–80 s, expected lifetime: 376 days, Vemco®, Nova Scotia, Canada) was implanted into the body cavity through a small mid-ventral incision anterior to the pelvic fins. The incision was closed by one or two separate sutures (Vicryl® absorbable, ETHICON, Johnson & Johnson, Medical Limited, Livingston, Scotland). Each pike was also floy-tagged for external identification; this tag was mounted with a thin metal wire through the dorsal muscle anterior to the dorsal fin. Total length and body mass were measured and a tissue sample (fin-clip) was stored in ethanol for genetic analysis. Sex was estimated from the external characteristics of the urinogenital papilla region. During surgery, the gills were flushed with lagoon water and after surgery pike were allowed to recover fully before being released into the lagoon at the site of capture.
To monitor pike movement in the lagoon, nine acoustic monitoring receivers (VR2; Vemco®, Nova Scotia, Canada) were deployed; six were placed to cover the main part of the lagoon, and three were placed at the outlet to determine movements in and out of the lagoon (Fig. 1). Detection range was tested to be between 100 and 500 m; hence, the receivers did not cover the full expanse of the lagoon. It was thus possible for a tagged fish to stay undetected in the lagoon, especially in the southern part of the lagoon where only a relatively small area was covered. The mouth of the lagoon was fully covered by receivers #7, #8 and #9 and all movement in and out was hence fully accounted for. Receiver data were offloaded every month during the study period from March 2003 to August 2004.
Spawning was assumed to take place between April and early May. In order to confirm spawning phenology, (untagged) pike were caught by rod on presumed spawning grounds in shallow waters on three occasions in April–May 2003 and 2004. Sex was determined from the external characteristics of the urinogenital papilla region, and the reproductive status (immature, ripe or spent) was evaluated by gently pressing the area of the gonads from the outside. All fish were subsequently released.
Water temperature was monitored by a Tidbit® temperature logger, placed in the harbour outlet (Fig. 1). Salinity was measured at least once a week, using a handheld refractometer and from August to October 2004 by use of a DST CTD logger (Star Oddi®, Iceland). Salinity measurements were taken in the harbour area at the mouth of the lagoon, where the salinity was expected to display maximum levels. In addition, salinity was measured using refractometer at six positions in the lagoon on regular occasions.
Data analyses
Data were analysed using software provided by the receiver manufacturer (VR2DATA, Vemco®, Nova Scotia, Canada). The data made it possible to establish a movement history for each fish and to determine if and when a fish left the lagoon and if and when it returned as well as how many days the pike was out of the lagoon. If a fish left the lagoon for more than one day, it was considered as ‘leaving the lagoon’. If it remained in the lagoon during the study period, it was classified as ‘resident’. Individual fish’s use of different parts of the lagoon during one year (1th April 2003–31th March 2004) was described by calculating the total number of days a pike was registered on a receiver (Reg. days). The data for each receiver were pooled to divide the lagoon into the north-eastern part (receivers #1, #2, #3), the middle part (receivers #4 and #5) and the southern part (receiver #6) as well as the harbour area (receivers #7, #8, #9).
Condition factor was calculated by use of Fultons K (Weatherley & Rogers, 1978). Probability of leaving the lagoon was modelled as a function of a common intercept (alpha) and the effects of length (TL), condition factor (K) and the two-way interaction between those and tested using binary logistic regression. The effect of length (TL) and condition factor (K) on total number of days out of the lagoon during a year was tested using linear regressions. Statistical analyses were done using the SPSS software package (IBM SPSS statistics 20).
Molecular analyses
Previously, the lagoon was stocked annually with pike fry from genetically differentiated non-native freshwater strains (Larsen et al., 2005). Between 1993 and 2006 in total 550,000 fry were stocked (Jacobsen et al., 2008). Microsatellite DNA analysis was therefore used to test the genetic origin of tagged individuals, using methods described in Bekkevold et al. (2014). Briefly, 12 microsatellite markers were analysed for all 22 tagged fish, and genotypes were compared with data from Bekkevold et al. (2014); specifically, two samples from the same location collected, respectively, in 1957 (i.e. before stocking) and in May 2007 (i.e. representing the spawning population after stocking had ceased), as well as samples from the three freshwater populations that had been used as broodstock (see Larsen et al., 2005), were used. Genetic differentiation among pairwise samples was estimated using Weir & Cockerham’s (1984) Theta, and statistical significance was estimated using Fisher’s exact tests, following Raymond & Rousset (1995). Genetic differences between ‘pike leaving the lagoon’ versus ‘resident pike’ (see above) were assessed using Discriminant Analyses of Principal Components (DAPC) implemented in the R package adegenet (Jombart et al., 2008) on genotypes from all Stege Lagoon collections (i.e. collected in 1956, 2007 and for the present study). First, the find.clusters() function was run for individual genotypes for K = 1–10. The best supported number of clusters was estimated comparing the Bayesian Information Criterion for the different values of K. Relationships among inferred clusters were then examined using the dapc() function. This function constructs synthetic variables, discriminant functions (DFs) that maximise variation between, while minimising variation within, groups and computes coordinates along these functions for each individual. The first 150 principle components (PCs) from the preliminary data transformation step were retained. From the derived DFs, posterior cluster membership probabilities were obtained for each individual genotype to the K clusters. DAPC was also used to visualise genetic relationships among samples.
Results
Salinity varied between 7 and 11 ppt in the lagoon during the study period, but was 8-10 ppt most of the time (Fig. 2a). Salinity measured at receiver positions in the lagoon varied in the same range, and salinity differences between the harbour and the most remote receivers ranged between 0 and 1.5 ppt (Fig. 2b) indicating that there was horizontally good mixing of the lagoon water.
Of the 22 tagged pike, 14 were identified as females (mean total length TL: 87.0 cm ± 9.7 SD, mean weight: 5.1 kg ± 1.7 SD) and eight as males (mean TL: 58.8 cm ± 5.9 SD, mean weight: 1.5 kg ± 0.4 SD) (see Table 1). Transmitter data were retrieved from all 22 pike for periods spanning 4–18 months (August 2004). The transmitter signal from one fish disappeared from the north-eastern part of the lagoon in July 2003 without registration in the central part of the lagoon or through the harbour outlet (ID 1). This could be due either to transmitter failure, tag loss, death of the fish with the tag buried in the sediment after a while or removal by a fisherman. Two fish were confirmed caught and killed by fishermen, one in the bay after four months (ID 7) and one in the lagoon after one year (ID 15). Finally, three fish left the lagoon without being registered in the lagoon again within the study period (ID 6, ID 9, ID 16, see below). At least one of these did return to the lagoon again without being registered by receivers probably due to transmitter failure or loss of battery power, since it was caught by angling in the lagoon in autumn 2004 (ID 9). On four occasions, tagged pike were caught and released by anglers or fishermen; of these, one was caught twice in 1 month.
Movements out of the lagoon
Fourteen pike (ten females, four males) left the lagoon during spring and summer 2003 (Fig. 3; Table 1), six returned between September and November 2003 and four in February through March 2004. The last four pike that left the lagoon were not recorded returning to the lagoon, two for known reasons (ID 7, ID 9, see above) and two with unknown fate (ID 6, ID 16). Most females left the lagoon between the end of April and the middle of May 2003, whereas the four males left the lagoon between the end of May and the beginning of August 2003. One female left the lagoon before spawning in 2003 (ID 20) but was on several occasions recorded in the harbour area outside the lagoon close to the outlet in late April. Eight pike, four females and four males, were only observed inside the lagoon (Fig. 3; Table 1). Of these fish, one was last recorded in the north-eastern part of the lagoon in July 2003 (ID 1) (see above). Hence, seven fish, three females and four males, were classified as residents, even though one of these fish (ID 2) left the lagoon at the end of March 2004.
By April 2004, more than 1 year after being tagged, 16 fish (73%) were recorded in the lagoon (Fig. 3). From late April to June 2004, nine fish (six females and three males) moved from the lagoon out to the bay again. Individual movement behaviour seemed to be consistent from year to year as the individuals that left the lagoon after spawning in 2004 corresponded with the individuals also leaving after the spawning season in 2003. Six of the resident fish were still in the lagoon after the spawning season 2004 and remained there until the end of the study period.
The ten pike that left the lagoon returned after 57 to 335 days during one year (Table 1). They made 2–5 excursions out of the lagoon during this year, eventually with short periods between excursions. Maximum duration of one excursion varied between individuals from 42 to 254 days, and minimum duration of an excursion varied between 1 and 60 days (Table 1). Movements out of the lagoon mainly took place during dawn and dusk, whereas return to the lagoon did not show any particular diel trend.
There was no significant effect of either condition factor K (P = 0.34) or TL (P = 0.34) at the time of tagging or the interaction between them (P = 0.28) on the individual probability of leaving the lagoon. For the ten fish that moved out and returned, there was a significant positive relationship between TL and the total number of days outside the lagoon (N = 10; R 2 = 0.41 P < 0.05; Fig. 4). There was no relationship between K and the total number of days outside the lagoon (N = 10; R 2 = 0.02, P = 0.73).
Movements in the lagoon
When in the lagoon, all but one tagged pike were registered in all four areas during the year from 1st April 2003 to 31st March 2004 (Fig. 5). The exception was pike ID 1 which disappeared in July 2003 (see above).
Number of daily registrations on receivers of each pike ID during one year (1. April 2003–31. March 2004). Registrations are divided into area of the lagoon: dark grey north-eastern part of the lagoon (receivers #1, #2, #3), medium grey middle part of the lagoon (receivers # 4, #5), pale grey southern part of the lagoon (receiver #6) and white harbour outlet area (receivers #7, #8, #9). Pike leaving the lagoon are marked with asterisk. Dead or disappeared pike during the 1-year period are marked with dagger
For the seven resident pike, the number of days per year with registrations on a receiver (Reg. days) was quite similar: mean 420 Reg. days ± 34.2 SD. Each pike was hence on average recorded on more than one (1.2) receiver per day, and pike were in general moving extensively in the lagoon. Pike that left the lagoon obviously had fewer registrations in the lagoon, and the number of registrations days varied corresponding to the variation in the time spent out of the lagoon.
Spawning period
In 2003 and 2004, respectively 26 and 39 pike were caught in the lagoon and their reproductive status was determined. On 23–24 April 1/3 of the pike were spent. A week later 2/3 were spent and on 5–6 of May all pike were spent (Fig. 6). This timing of spawning activity was the same both years (Fig. 6). Almost all catches were females and only three males were caught, all in slightly deeper areas.
Genetic assignment analyses
Genotypes were recorded successfully for all individuals and loci. Estimates of sample differentiation showed close, and statistically not significantly divergent, genetic relationship between tagged pike and the Stege Lagoon sample collected in 2007 (Online resource 1, Table S1). This was also reflected in the DAPC analysis. Three PCs, respectively, explaining 0.46, 0.28 and 0.26 of the variation, were retained in the analysis and K = 4 clusters had the highest probability. All collections from Stege Lagoon grouped together in a single cluster, and the three broodstock populations grouped in three clusters of their own (Online resource 1, Fig. S1). Posterior cluster membership probabilities for tagged fish indicated that they belonged to the Stege Lagoon population (all membership probabilities >0.9). DAPC analysis incorporating only the 104 Stege samples did not reveal any clustering of genotypes according to moving behaviour (not shown), again indicating that residents and pike leaving the lagoon belonged to the same genetic population.
Discussion
Pike living on the edge
This is to our knowledge the first study to report on behaviour of adult pike living in salinities up to 11 ppt. Previous studies on brackish water pike are from populations in the eastern and northern Baltic Sea (Müller, 1986; Karås & Lethonen, 1993; Westin & Limburg, 2002; Engstedt, 2011), and from the Caspian Sea (Stolyarov & Abusheva, 1997), where salinity never exceeds 6–7 ppt. Some of these pike migrate to freshwater spawning sites in spring, but coastal spawning is also reported. Often, populations with different spawning strategies coexist (Westin & Limburg, 2002; Engstedt, 2011). Westin & Limburg (2002) showed that pike spawning on the brackish coast of Gotland had eggs that developed normally at slightly higher salinities (6.9 ppt) than the sympatric population of pike, migrating to spawn in freshwater, whose eggs were not able to develop at above 6.0 ppt. This suggests local adaptation to spawning in brackish water, although maternal and other non-genetic effects could play a role. In the present study, all tagged pike were recorded in the lagoon during the spawning period, and none migrated into the Lendemark Brook, which constitutes the only freshwater habitat in the area. Thus, it is concluded that the pike did spawn in the brackish water of the lagoon. The present study gave no indications of whether the spawning resulted in viable eggs, larvae and juveniles. However, the genetic analyses reported here and in Larsen et al. (2005) strongly suggest that pike in the lagoon were self-sustaining, i.e. that successful recruitment occurs at a regular level. A subsequent study from this lagoon showed that fertilisation and development of eggs was successful at 8.5 ppt, but did not address higher levels of salinity (Jørgensen et al., 2010). Pike larvae from the lagoon exhibited normal development and behaviour at salinities up to 13.5 ppt, above which stress behaviour and reduced growth were observed (Jørgensen et al., 2010). Spawning and egg and larvae development are therefore likely to have been successful during 2002–2005, when salinity was measured in the lagoon. However, the salinity data also show that although salinity fluctuates between 7 and 11 ppt most of the time, substantial inflow of seawater can occur. This, e.g., resulted in 17 and 20 ppt. being measured on one occasion in the harbour area in January 2003 and 12–16 ppt in January 2005 (see Fig. 2a). There are no measures from more remote areas in the lagoon during periods of highly saline inflows, but it is reasonable to believe that the salinity in the lagoon is also influenced at least when salinity in the outlet is high for longer periods, since measurements during other periods revealed a maximum difference of only 1.5 ppt. between the harbour and the north-eastern part of the lagoon. This suggests that pike in the lagoon, and especially those that move out of the lagoon, are in fact living on the edge of their physiological limit and therefore from time to time likely experience severe salt stress or even local population crashes, as documented in Dahl (1961). The relatively high salinity tolerance in pike from brackish areas like Stege Lagoon is likely a result of local adaptation; the salinity tolerance of local pike larvae from the lagoon (Jørgensen et al., 2010) was approximately 2 ppt. higher compared to a previous study of pike larvae of freshwater origin (Jacobsen et al., 2007). The direct environmental exposure experienced by individual pike could be measured by taking advantage of the rapid development in aquatic telemetry and tagging pike with telemetry tags equipped with a conductivity sensor (Hussey et al., 2015).
Movements out of the lagoon
In general, we see two movement patterns, i.e. the majority of the tagged pike left the lagoon for longer periods soon after spawning, whereas a third of the fish were resident in the lagoon. All registered pike repeated their movement pattern in both years, suggesting individual behavioural consistency as recently reported for migrating Baltic pike (Tibblin et al., 2016) as well as for pike in lakes and other fishes (Jepsen et al., 2001; Brodersen et al., 2012; Aarestrup et al., 2015). Since pike leaving the lagoon returned before spawning the subsequent year, it seems clear that the lagoon also functions as spawning ground for the part of the population that left the lagoon. There was no indication of major differences in average salinity between the lagoon and the neighbouring bays and coastal areas in the study period. Despite the incidents of inflows of more saline water at other times of the year, salinity may nonetheless be more stable in the lagoon during spawning compared to more exposed locations outside the lagoon. Whether pike spawning also takes place outside the lagoon was not addressed in the present study, since we only tagged pike caught in the lagoon immediately prior to spawning. One fish (ID 20), though, left the lagoon by the end of March 2003 (i.e. presumably prior to spawning) and was often registered just outside the harbour area during the spawning time. Thus, the spawning area of this fish is not clear. The fates of two fish not registered as returning to the lagoon are unknown; these fish may have had different movement behaviour without homing, but this could also be due to death or transmitter malfunction.
Individuals that left the lagoon did eventually return to the lagoon for shorter periods, but they were all away from the lagoon for at least one extensive period lasting between one to more than eight months. This indicates longer expeditions to more remote areas and not just short accidental transits in and out of the narrow harbour outlet. There was no evidence that pike exhibiting different movement patterns belonged to genetically differentiated populations. Although divergent migratory phenotypes may have a heritable component, the analysis showed that the different migratory phenotypes are contained within the same population. In studies of partial migration (Jonsson & Jonsson, 1993; Skov et al., 2008; Chapman et al., 2012), the motivation for some individuals of a population to move to other areas has been linked to condition factor and a trade-off between predation risk and feeding (Brönmark et al., 2008). In our study, there was no obvious predation risk for the pike from other fish, except for cannibalism from larger conspecifics. A potential risk could be from seals, e.g. harbour seal (Phoca vitulina L.), but there are no reports on this. There is no reason why predation risk from other pike would be lower in the lagoon compared to the bay; on the contrary, the high density of pike in the lagoon could impose some risk for smaller pike. So the winter movements into the lagoon are more likely linked to spawning and/or overwintering in a less exposed environment, than avoiding predation. Likewise, there was no indication that movement behaviour was associated with size-related predation risk, since size had no influence on the probability of leaving the lagoon. In addition, the smallest of the pike left the lagoon for the briefest periods. Motivation for leaving the lagoon might instead be associated with exploiting alternative food resources outside the lagoon during summer, following ‘ideal free distribution’ principles (Wootton, 1998). Returning to the lagoon in autumn could then correspond with a response to decreasing temperatures and lower food demands. Among pike that left the lagoon, the largest individuals stayed longer outside the lagoon. This could indicate a size effect on tolerance to a more exposed environment in the bay, or that the larger individuals undertake more extensive movements once they leave the lagoon simply because they are faster swimmers with a smaller per capita cost of swimming and hence capable of longer distance forays. However, the latter explanation remains unsupported by the present study as no data on the extent of movement outside the lagoon were collected.
The two movement patterns observed can be considered to reflect a resident group with a restricted home range and a group that leaves the lagoon for longer periods and has a more extensive home range. Alternatively, the movement activity may also have a continuous distribution, with individual variation in home range. Regardless of the patterns of distribution, it may follow ‘ideal free’ principles and depend on resource distribution between the different habitats (Wootton, 1998) in order to maximise fitness as demonstrated for pike in two connected lake basins (Haugen et al., 2006). If so, it is interesting that individuals seemed to have a fixed affinity for either moving outside the lagoon or not, i.e. that ideal free distribution can occur in a way that is temporally consistent between individuals. Traditionally, pike has been considered to be mainly stationary with a restricted home range outside of spawning migrations (Craig, 1996). However, there is growing evidence from studies using active telemetry and other methods monitoring individual swimming behaviour that pike movements can be unpredictable and extensive (Jepsen et al., 2001; Koed et al., 2006; Knight et al., 2008; Baktoft et al., 2012). This is supported by the present study, showing that pike in a 5 km2 large brackish area utilise the entire lagoon both during a year and regularly on shorter time scales (days to months), suggesting larger and more overlapping home ranges than might be expected by a stationary predator.
Implications for management
In most areas of the Baltic Sea including the Danish straits, commercial catches of pike have declined considerably since the 1970s–1980s suggesting a drastic population decrease (Nilsson et al., 2004; Nilsson, 2006; Lehtonen et al., 2009; Ljungren et al., 2010). Reasons for these declines in the different areas are not well determined, whether due to poor recruitment, habitat deterioration, eutrophication or other reasons. There is increasing interest in pike angling, not least in brackish areas and thus increasing socioeconomic potential of brackish water pike. Hence, there is a demand for accomplished management of these fish and not the least for identification of management units. The present study adds to the knowledge of pike living on the edge of their salinity tolerance and gives evidence that besides freshwater inlets (Engstedt, 2011), protected brackish areas may be much more important for recruitment than previously thought. Our study also shows that successful recruitment may hinge on unhindered access to lagoons and other protected areas, and that intense fisheries exploitation at lagoon mouths in particular during periods of movements renders the populations particularly vulnerable. Thus, temporal and spatial fisheries regulations are likely to be a valuable measure to protect and increase these populations.
References
Aarestrup, K., H. Baktoft, E. B. Thorstad, J. C. Svendsen, J. Höjesjö & A. Koed, 2015. Survival and progression rates of anadromous brown trout kelts Salmo trutta during downstream migration in freshwater and at sea. Marine Ecology Progress Series 535: 185–195.
Baktoft, H., K. Aarestrup, S. Berg, M. Boel, L. Jacobsen, N. Jepsen, A. Koed, J. C. Svendsen & C. Skov, 2012. Seasonal and diel effects on the activity of northern pike studied by high-resolution positional telemetry. Ecology of Freshwater Fish 21: 386–394.
Bekkevold, D., L. Jacobsen, J. Hemmer-Hansen, S. Berg & C. Skov, 2014. From regionally predictable to locally complex population structure in a freshwater top predator: river systems are not always the unit of connectivity in Northern Pike Esox lucius. Ecology of Freshwater Fish 24: 305–331.
Brodersen, J., P. A. Nilsson, B. B. Chapman, C. Skov, L.-A. Hansson & C. Brönmark, 2012. Variable individual consistency in timing and destination of winter migrating fish. Biology Letters 8: 21–23.
Brönmark, C., C. Skov, J. Brodersen, P. A. Nilsson & L.-A. Hansson, 2008. Seasonal migration determined by a trade-off between predator avoidance and growth. PLoS One 3: e1957.
Chapman, B. B., K. Hultén, J. Brodersen, P. A. Nilsson, C. Skov, L.-A. Hansson & C. Brönmark, 2012. Partial migration in fishes: causes and consequences. Journal of Fish Biology 81: 456–478.
Craig, J. F., 1996. Pike: Biology and Exploitation. Chapman & Hall, London.
Dahl, J., 1961. Alder og vækst hos danske og svenske brakvandsgedder. Ferskvandsfiskeribladet 59: 34–38. (in Danish).
Engstedt, O., 2011. Anadromous pike in the Baltic Sea. PhD Dissertation, Linnaeus University, Kalmar, Sweden.
Fischer, H. & W. Matthäus, 1996. The importance of the Drogden Sill in the Sound for major Baltic inflows. Journal of Marine Systems 9: 137–157.
Haugen, T. O., I. J. Winfield, L. A. Vøllestad, J. M. Fletcher, J. B. James & N. C. Stenseth, 2006. The ideal free pike: 50 years of fitness-maximizing dispersal in Windermere. Proceedings of the Royal Society B 273: 2917–2924.
Hussey, N. E., S. T. Kessel, K. Aarestrup, S. J. Cooke, P. D. Cowley, A. T. Fisk, R. G. Harcourt, K. N. Holland, S. J. Iverson, J. F. Kocik, J. E. M. Flemming & F. G. Whoriskey, 2015. Aquatic animal telemetry: a panoramic window into the underwater world. Science 348: 1221–1231.
Jacobsen, L., C. Skov, A. Koed & S. Berg, 2007. Short-term salinity tolerance of northern pike, Esox lucius, related to temperature and size. Fisheries Management and Ecology 14: 303–308.
Jacobsen, L., C. Skov, S. Berg, A. Koed & P. F. Larsen, 2008. Udsætning af geddeyngel som bestandsophjælpning i danske brakvandsområder – effektvurdering og perspektivering. Technical University of Denmark, DTU Aqua report no. 196-08. ISBN 978-87-7481-085-8. 54 p. (in Danish).
Jepsen, N., S. Beck, C. Skov & A. Koed, 2001. Behaviour of pike (Esox lucius L.) >50 cm in a turbid reservoir and in a clearwater lake. Ecology of Freshwater Fish 10: 26–34.
Jombart, T., 2008. ADEGENET: an R package for the multivariate analysis of genetic markers. Bioinformatics 24: 1403–1405.
Jonsson, B. & N. Jonsson, 1993. Partial migration: niche shift versus sexual maturation in fishes. Reviews of Fish Biology and Fisheries 3: 348–365.
Jørgensen, A. T., B. W. Hansen, B. Visman, L. Jacobsen, C. Skov, S. Berg & D. Bekkevold, 2010. High salinity tolerance in eggs and fry of a brackish Esox lucius population. Fisheries Management and Ecology 17: 554–560.
Karås, P. & H. Lehtonen, 1993. Patterns of movement and migration of pike (Esox lucius L.) in the Baltic Sea. Nordic Journal of Freshwater Research 68: 72–79.
Knight, C. M., R. E. Gozlan & M. Lucas, 2008. Can seasonal home-range size in pike Esox lucius predict excursion distance? Journal of Fish Biology 73: 1058–1064.
Koed, A., K. Balleby, P. Mejlhede & K. Aarestrup, 2006. Annual movements of adult pike (Esox lucius L.) in a lowland river. Ecology of Freshwater Fish 15: 191–199.
Larsen, P. F., M. M. Hansen, E. E. Nielsen, L. F. Jensen & V. Loeschcke, 2005. Stocking impact and temporal stability of genetic composition in a brackish northern pike population (Esox lucius L.), assessed using microsatellite DNA analysis of historical and contemporary samples. Heredity 95: 136–143.
Lehtonen, H., E. Leskinen & R. Selén, 2009. Potential reasons for the changes in the abundance of pike, Esox lucius, in the western Gulf of Finland, 1939-2007. Fisheries Management and Ecology 16: 484–491.
Ljungren, L., A. Sandström, U. Bergström, J. Mattila, A. Lappalainen, G. Johnasson, G. Sundblad, M. Casini, O. Kaljuste & B. K. Erikson, 2010. Recruitment failure of coastal predatory fish in the Baltic Sea coincident with an offshore ecosystem regime shift. ICES Journal of Marine Sciences 67: 1587–1595.
Müller, K., 1986. Seasonal anadromous migration of the pike (Esox Lucius L.) in coastal areas of the northern Bothnian Sea. Archive für Hydrobiologie 107: 315–330.
Nilsson, J., 2006. Predation of northern pike (Esox lucius L.) eggs: a possible cause of regionally poor recruitment in the Baltic. Hydrobiologia 553: 161–169.
Nilsson, J., J. Andersson, P. Karås & O. Sandström, 2004. Recruitment failure and decreasing catches of perch (Perca fluviatilis L.) and pike (Esox lucius L.) in coastal waters of southeast Sweden. Boreal Environmental Research 9: 295–306.
Raymond, M. & F. Rousset, 1995. An exact test for population differentiation. Evolution 49: 1283–1286.
Rothla, M., M. Vetemaa, K. Urtson & A. Soesoo, 2012. Early life migration of Baltic Sea pike Esox lucius. Journal of Fish Biology 80: 886–893.
Skov, C., J. Brodersen, P. A. Nilsson, L.-A. Hansson & C. Brönmark, 2008. Inter- and size-specific patterns of fish seasonal migration between a shallow lake and its streams. Ecology of Freshwater Fish 17: 406–415.
Stolyarov, I. A. & K. Abusheva, 1997. Pike Esox lucius of Kizlyar Bay of the northern Caspian Sea. Journal of Ichtyology 37: 268–271.
Tibblin, P., A. Forsman, T. Borger & P. Larsson, 2016. Causes and consequences of repeatability, flexibility and individual fine-tuning of migratory timing in pike. Journal of Animal Ecology 85: 136–145.
Weir, B. S. & C. C. Cockerham, 1984. Estimating F-statistics for the analysis of population structure. Evolution. 38: 1358–1370.
Westin, L. & K. E. Limburg, 2002. Newly discovered reproductive isolation reveals sympatric populations of Esox lucius in the Baltic. Journal of Fish Biology 61: 1647–1652.
Weatherley, A. H. & S. C. Rogers, 1978. Some aspects of age and growth. In Gerking, S. D. (ed.), Ecology of Freshwater Fish Production. Blackwell Scientific Publications, Oxford: 52–74.
Wootton, R. J., 1998. Ecology of Teleost Fishes. Kluwer Academic Publishers, Dordrecht.
Acknowledgments
We gratefully acknowledge the help with catching, tagging and tracking pike from Morten Carøe, Hans-Jørn Christensen, Jørgen Skole Mikkelsen, Michael Gramkov and Kim Iversen. We sincerely thank Peter Gruth Hansen, Kurt Schierup, Bo Skat Riese and Bent Hjort for salinity measurements. Noor de Jong performed microsatellite analyses. The Danish Angling License Funds is thanked for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Odd Terje Sandlund
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jacobsen, L., Bekkevold, D., Berg, S. et al. Pike (Esox lucius L.) on the edge: consistent individual movement patterns in transitional waters of the western Baltic. Hydrobiologia 784, 143–154 (2017). https://doi.org/10.1007/s10750-016-2863-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2863-y