Abstract
The comprehension and usage of primate alarm calls appear to be influenced by social learning. Thus, alarm calls provide flexible behavioral mechanisms that may allow animals to develop appropriate responses to locally present predators. To study this potential flexibility, we compared the usage and function of 3 alarm calls common to 2 closely related sifaka species (Propithecus verreauxi and P. coquereli), in each of 2 different populations with different sets of predators. Playback studies revealed that both species in both of their respective populations emitted roaring barks in response to raptors, and playbacks of this call elicited a specific anti-raptor response (look up and climb down). However, in Verreaux’s sifakas, tchi-faks elicited anti-terrestrial predator responses (look down, climb up) in the population with a higher potential predation threat by terrestrial predators, whereas tchi-faks in the other population were associated with nonspecific flight responses. In both populations of Coquerel’s sifakas, tchi-fak playbacks elicited anti-terrestrial predator responses. More strikingly, Verreaux’s sifakas exhibited anti-terrestrial predator responses after playbacks of growls in the population with a higher threat of predation by terrestrial predators, whereas Coquerel’s sifakas in the raptor-dominated habitat seemed to associate growls with a threat by raptors; the 2 other populations of each species associated a mild disturbance with growls. We interpret this differential comprehension and usage of alarm calls as the result of social learning processes that caused changes in signal content in response to changes in the set of predators to which these populations have been exposed since they last shared a common ancestor.
Similar content being viewed by others
Introduction
Studies of animal communication aim to identify the relation between signals and associated events to infer the potential meaning and function of signals. Most animals seem to communicate about immediate behavior, thereby conveying information about their affective state, but sometimes also transmitting information about specific external objects or events, as in functionally referential predator alarm calls (Fichtel et al. 2001; Marler et al. 1992; Seyfarth and Cheney 2003; Struhsaker 1967). Much recent research in animal communication has focused on intra- and interpopulation comparisons to investigate whether signals are subject to modification and social transmission (Fragaszy and Perry 2003; Janik and Slater 2003; Marler and Slabbekoorn 2002). In nonhuman primate communication, gestural signals seem to be more flexible and subject to cultural transmission than facial or vocal signals (Cheney and Seyfarth 2007; Pollick and de Waal 2007; van Schaik et al. 2003; Whiten et al. 1999).
In the domain of primate vocal communication, learning can influence the production, usage, or comprehension of vocalizations (Janik and Slater 2003; Seyfarth and Cheney 1997, 2010). First, it is generally assumed that the production of the basic acoustic structure of vocalizations is predominately genetically determined, but subtle acoustic variation in calls between groups can arise as a result of social learning (Crockford et al. 2004; Hammerschmidt et al. 2001; Snowdon 2001; Winter et al. 1973). Second, the ability to use vocalizations in an appropriate context appears to be partly innate and partly socially learned, and thus more flexible (Hauser 1988; Seyfarth and Cheney 1997). For example, infant vervets (Chlorocebus aethiops) produce an alarm call inappropriately upon detecting nonthreatening species such as pigeons or warthogs. Although they do give eagle alarm calls only to birds, and leopard alarm calls only to terrestrial mammals, they learn to discriminate between nonpredatory and predatory birds by observation of anti-predator behavior of other group members (Seyfarth and Cheney 1986). Third, call comprehension appears to be more flexible and influenced by experience and learning than call production and usage (Seyfarth and Cheney 2010). For example, young vervets or Verreaux’s sifakas (Propithecus verreauxi) need experience before they respond appropriately to their own or other species’ alarm calls, and are likely to learn by observation of adult responses (Fichtel 2008; Hauser 1988; Seyfarth and Cheney 1997). Moreover, studies focusing on the acquisition of predator evasion tactics in rhesus monkeys (Macaca mulatta), as well as in birds and marsupials such as naïve blackbirds (Turdus merula) or tammar wallabies (Macropus egenii), revealed that fear responses could be conditioned to formerly novel objects by observing conspecifics showing fear or mobbing responses toward the object (Cook and Mineka 1989; Curio et al. 1978; Griffin and Evans 2003). These experiments indicate that the kinds of stimuli eliciting the response are learned and based on observations of the adults’ responses. Thus, observation-based conditioning in usage and comprehension of alarm calls potentially provides animals the flexibility to develop appropriate responses to a local set of predators (Cook and Mineka 1989; Curio et al. 1978; Fichtel and van Schaik 2006; Laland 2004).
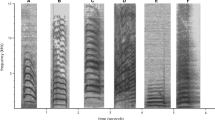
We investigated the flexibility in comprehension of alarm calls in 2 closely related Malagasy primates, Verreaux’s sifakas (Propithecus verreauxi) and Coquerel’s sifakas (P. coquereli) (Pastorini et al. 2001). Earlier studies of a wild population of Propithecus verreauxi in Kirindy forest in western Madagascar and a population of semifree-ranging P. coquereli at the Duke Lemur Center (Durham, NC) revealed that both species produce similar alarm calls but exhibit differences in usage and, most strikingly, in comprehension of alarm calls (Table I; Fichtel and Kappeler 2002; Fichtel and van Schaik 2006). Specifically, after detection of aerial predators, both species look skywards, climb down, and produce roaring barks, which are sometimes followed by growls (Fig. 1). Both species also produce growls during aggressive interactions between conspecifics. Interestingly, after experimental playbacks of growls, verreauxi seem to associate the presence of a terrestrial predator with them, whereas coquereli responded to growls as if they indicated the presence of a raptor (look skywards, climb down, emit roaring barks). Because coquereli in the outdoor enclosures are mainly exposed to aerial predators, they may have associated 2 alarm calls—roaring barks and growls—with the presence of aerial predators (Fichtel and van Schaik 2006). After detecting terrestrial predators, both species look toward the ground and climb up, and verreauxi produce growls whereas coquereli produce tchi-faks in this situation (Fig. 1). However, verreauxi produce tchi-faks only when a fossa (Cryptoprocta ferox) directly attacks and chases them. In addition, both species produce tchi-faks in response to lost calls of group members, when their group is widely spread, and during intergroup encounters (Fichtel and Kappeler 2002; Fichtel and van Schaik 2006).
Because the results of these 2 studies indicated that there might be variation in usage and comprehension of structurally similar alarm calls between these 2 closely related species, we expanded this research project by studying these alarm calls in additional wild populations. To study whether the usage and comprehension of alarm calls is more likely to be innate or learned, we examined alarm call behavior by contrasting populations of each species with different sets of predators. If usage and comprehension of alarm calls are influenced primarily by innate predispositions, we would expect the 2 closely related species, and certainly members of the same species from different populations, to exhibit very similar behavioral patterns in this context. If the usage and comprehension are due to differences in predator exposure history and reflect the different outcomes of developmental processes used to acquire the association of a call with its referent, we would expect variation in alarm call behavior. Because the usage and comprehension of growls and tchi-faks seemed to vary most strikingly, we examined whether these calls are more likely to be associated with terrestrial predators by contrasting the usage and comprehension of these calls in populations with different densities of terrestrial predators. Because Propithecus verreauxi was previously studied in Kirindy, where they are exposed to a high density of terrestrial predators (Fichtel and Kappeler 2002), we predicted that verreauxi at Berenty, where they are practically not exposed to terrestrial predators, would exhibit a less specific usage and comprehension of growls and tchi-faks. Because coquereli in the outdoor enclosure were mainly exposed to aerial, but to virtually no terrestrial predators, we predicted that wild coquereli at the Parc National Ankarafantsika, where they are also exposed to terrestrial predators, would not associate growls with aerial predators.
Methods
Study Site and Subjects
Propithecus verreauxi
At Kirindy Forest, Propithecus verreauxi are individually marked as part of a long-term study. Depending on the playback stimulus, we studied 8–10 adults from 6–8 different social groups (Fichtel and Kappeler 2002). At Kirindy, verreauxi are exposed to Harrier hawks (Polyboroides radiuatus), a high density of the fossa, and stray dogs, all of which are known sifaka predators (Dollar et al. 2007; Goodman 2003; Hawkins and Racey 2008; Karpanty and Goodman 1999).
At Berenty Reserve, we studied individuals from 11 different social groups that were distinguished by their size, sex ratio, and home range location. We recognized individuals through earmarks, scratches, or distinctive fur coloration. We chose 11 easily recognizable adults (7 males and 4 females) as experimental subjects. At Berenty, verreauxi are exposed to the Harrier hawk, but the fossa has been absent since at least 1975 (A. Mertl-Millhollen pers. comm.). Only cattle herders’ dogs occasionally sneak into the reserve, but attacks on sifakas have not been observed (A. Mertl-Millhollen pers. comm.). In both habitats, Propithecus verreauxi have been habituated to the presence of humans.
Propithecus coquereli
At the Duke Lemur Center (DLC), we studied 2 semifree-ranging groups that were housed in 2 different natural-habitat enclosures (NHE3 and NHE6) during the summer (April–October), with sizes of 1.5 and 4 ha, respectively. Both enclosures are dominated by mixed pine and hardwood forest. Although coquereli were provisioned daily with fruit, vegetables, and monkey chow, they spent much of their time foraging on the local arboreal flora. Regularly seen potential predators include red-tailed hawks (Buteo jamaicensis) and foxes (Vulpes vulpes). However, only attacks by red-tailed hawks have ever been observed, (D. Haring pers. comm.). The 2 groups of coquereli contained 4 and 5 individuals, respectively, that were individually recognizable through earmarks, scratches, or distinctive fur coloration. Individuals belonged to the first or second generation of the wild-caught founder generation, and in 1 group a wild-caught male was still present. We conducted experiments with 4 adult females and 4 adult males of these groups.
We studied wild coquereli within the tourist area of Ankarafantsika National Park, where several groups have been well habituated to the presence of humans. The fossa, which are the most important predators of sifakas (Hawkins and Racey 2008; Lührs and Dammhahn 2010), stray dogs, and the Harrier hawk occur at this site. We studied 8 different social groups that were distinguished by their size, sex ratio, and home range location. We could also individually recognize the sifakas through earmarks, scratches, lost fingers, or distinctive fur coloration. We chose 10 easily recognizable adult individuals (7 males and 3 females) as experimental subjects.
Playback Stimuli
We made playback stimuli by recordings of natural sifaka alarm calls and alarm calls given in response to playbacks of predator vocalizations (Fichtel and Kappeler 2002; Fichtel and van Schaik 2006). We recorded alarm calls with a SONY, DCR-PC100E digital video camera (frequency response: 60–16,000 Hz) or a Marantz solid-state recorder PMD 660 (frequency response: 40–20,000 Hz) and a Sennheiser directional microphone K6 power module and ME66 recording head (frequency response: 40–20,000 Hz) with MZW66 pro windscreen. Because sifakas usually produce several bouts of alarm calls in response to predators, each playback stimulus was repeated 3 times with intervals of 5 s silence in between, using Cool Edit 2000 (Syntrillium, Phoenix, AZ). We tested individuals with playback experiments of tchi-faks, growls, and roaring barks that we recorded at the same site. To avoid pseudoreplication, we used alarm calls from a different adult individual for each playback experiment.
Playback Procedures
We played back vocalizations with a Sony WM TCD-100 DAT recorder and a NAGRA DSM amplifier-loudspeaker (frequency response: 60–15,000 Hz) hidden behind a bush or tree at a distance of ≥5 m from the focal individual. We adjusted the sound pressure level to 70 ± 1 dB measured at a distance of 3 m (sound level meter, fast weighting, Voltcraft 320). The playback procedure was the same in all populations: We searched for the group, identified all group members, and waited 30–50 min until the subjects were used to our presence before starting the playback experiment. We presented alarm calls only when individuals were at an intermediate elevation where they had the possibility to climb either up or down or out of the tree. We chose only individuals that were engaged in relatively quiet activities, such as foraging, resting, or grooming as focal subjects. We conducted playback experiments in each group with ≥1 d but on average 2–3 d (wild populations) and 3 d (captive populations) between presentations in a randomized but counterbalanced order. We videotaped 1 focal individual with a Sony digital video camera connected to the directional microphone for 1–2 min after the onset of each playback.
Data Analyses
Analyses of the behavior of focal individuals were based on data obtained from the video recordings, using a frame-by-frame analysis with a resolution of 25 frames/s (using iMovie, Macintosh). To calibrate the onset of the playback experiments, we used SPARK audio-processing LE 2.6. Without knowing the type of playback beforehand, C. Fichtel scored occurrence, frequency, or duration of the following responses within the first minute after the onset of a playback: 1) Vocalization: occurrence; if yes, call type (frequency); 2) Look: duration of looking in different directions (up or down, duration); 3) Escape: subject climbed up or down. We scored looking directions by head movements of the focal subject that deviated by 45° from an imaginary horizontal line drawn from the center of the head. We compared the distribution of vocal and escape responses between the populations of the same species via a Fisher’s exact test. We compared variation between populations in looking responses via a Mann-Whitney U-test.
Results
Roaring Barks
After the presentation of roaring barks, 63% of verreauxi at Kirindy and 91% of those at Berenty responded with roaring barks; 9% of verreauxi at Berenty also emitted growls (Fig. 1; Table II). The frequency of observed alarm calls after roaring bark playbacks did not differ between the 2 populations (2-tailed Fisher’s exact: roaring barks: p = 0.262; growls: p = 1.0). Playback experiments with roaring barks led 63% of verreauxi at Kirindy and all individuals at Berenty to climb down (Fig. 2a; 2-tailed Fisher’s exact: p = 0.058). At Kirindy and Berenty, verreauxi also did not differ in looking duration after the broadcast of roaring barks (Fig. 3a; Mann-Whitney U-test: roaring bark: n 1 = 8, n 2 = 11, Z = −1.651, p = 0.109).
In response to roaring bark playbacks, most coquereli responded with roaring barks (captivity = 100%, wild = 80%) and 38% of captive and 40% of wild coquereli responded additionally with growls at the end of the roaring bark bout. Only 10% of wild individuals also gave tchi-faks after roaring barks. The observed frequencies of alarm calls did not differ between wild and captive populations (Table II; 2-tailed Fisher’s exact: roaring barks: p = 0.477, growls: p = 1.0, tchi-faks: p = 1.0). In response to roaring bark playbacks, 63% of captive and 60% of wild individuals climbed down (Fig. 2b; 2-tailed Fisher’s exact: p = 1.0). The presentation of roaring barks led coquereli of both populations to look skywards, with wild coquereli looking much longer (Fig. 3b, Mann-Whitney U-test: looking down: n 1 = 8, n 2 = 8, Z = −1.365, p = 0.195, looking up: n 1 = 8, n 2 = 10, Z = −2.578, p = 0.009).
Growls
Playback experiments with growls elicited growls significantly less often in verreauxi at Kirindy (0%) than in those (45%) at Berenty (Fig. 1; Table II; 2-tailed Fisher’s exact: p = 0.045). Startle responses after growl playbacks also differed between the populations: at Kirindy 50% of individuals climbed up, whereas at Berenty 27% of individuals climbed up and 45% of individuals climbed down (Fig. 2a; 2-tailed Fisher’s exact: climbing up: p = 0.377, climbing down: p = 0.045). Looking duration also differed between populations after presentation of growls, with verreauxi at Kirindy spending more time looking down (Fig. 3a, Mann-Whitney U-test: growl: n 1 = 8, n 2 = 11, Z = −2.53, p = 0.009).
Interestingly, after the broadcast of growls, 100% of captive coquereli responded with roaring barks and 63% of them also emitted growls. In contrast, 40% of wild coquereli responded only with growls and 10% of individuals with tchi-faks (Table II; 2-tailed Fisher’s exact: roaring barks: p = 0.001, growls: p = 0.637, tchi-faks: p = 1.0). After the presentation of growls, 63% of captive coquereli climbed down, whereas only 10% of wild coquereli climbed up (Fig. 2b; 2-tailed Fisher’s exact: climbing down: p = 0.007, climbing up: p = 1.0). Average looking duration also differed between populations, with captive coquereli looking for much longer skywards, whereas wild coquereli looked downwards for much longer, indicating that captive coquereli associated the presence of raptors with these calls, whereas wild coquereli associated a nonspecific disturbance with them (Fig. 3b, Mann-Whitney U-test: looking down: n 1 = 8, n 2 = 10, Z = −2.934, p = 0.002, looking up: n 1 = 8, n 2 = 10, Z = −2.791, p = 0.004).
Tchi-faks
In response to playback experiments with tchi-faks, 60% of verreauxi at Kirindy responded with growls, whereas 9% of verreauxi at Berenty responded with a tchi-fak (Fig. 1; Table II; 2-tailed Fisher’s exact: tchi-faks: p = 1.0, growls: p = 0.004). After playback experiments with tchi-faks, verreauxi also showed divergent escape responses, with 50% of verreauxi at Kirindy climbing down, whereas only 18% of individuals at Berenty climbed down and 45% climbed up (Fig. 2a; 2-tailed Fisher’s exact: climbing down: p = 1.0, climbing up: p = 0.035). Average duration of looking up or down did not differ between populations after presentation of tchi-faks (Fig. 3a, Mann-Whitney U-test: n 1 = 10, n 2 = 11, Z = −1.831, p = 0.072).
In coquereli, tchi-fak playbacks elicited the same call in all captive and 50% of wild coquereli, with captive individuals responding significantly more often (Fig. 1; Table II; 2-tailed Fisher’s exact: p = 0.036). Playback experiments with tchi-faks elicited similar escape responses by leading 38% of captive and 50% of wild coquereli to climb up (Fig. 2b; 2-tailed Fisher’s exact: p = 0.664). Captive and wild coquereli also did not differ in time spent looking up or down after the broadcast of tchi-faks (Fig. 3b, Mann-Whitney U-test: looking down: n 1 = 7, n 2 = 10, Z = −0.293, p = 0.813, looking up: n 1 = 8, n 2 = 10, Z = −0.711, p = 0.515).
Discussion
Our results revealed that both Propithecus verreauxi and P. coquereli produced roaring barks in response to aerial predators and exhibited similar escape strategies with these alarm calls. However, the relative usage and function of growls and tchi-faks differed slightly between species and more strikingly within them.
Roaring Barks
In all populations, verreauxi and coquereli exhibited similar responses to roaring barks by emitting roaring barks, climbing down, and looking skywards (Table I). Because in all populations raptors were common, sifakas clearly associate the presence of a raptor with these calls.
Growls
In verreauxi, growls elicited different escape responses in the 2 populations: Kirindy verreauxi spent more time looking down than Berenty verreauxi and some Kirindy verreauxi climbed up, whereas Berenty verreauxi either climbed up or down. Growls were given in both populations after bouts of roaring barks and during social disturbances. In addition, Kirindy verreauxi also emitted growls upon detecting stray dogs whereas Berenty verreauxi produced tchi-faks upon detecting small terrestrial predators (snakes and a small viverrid, Table I). We propose that these differences are linked to the presence of particular local predators at each site. At Kirindy, fossas occur at relatively high densities (Hawkins and Racey 2008; Lührs and Kappeler, unpubl. data), and stray dogs pass through the forest occasionally. There are also several large snakes, but sifakas have not been observed to show any anti-predator behavior in response to snakes during 15 yr of observations. At Berenty, the fossa has been absent at least since 1975 and stray dogs are extremely rarely seen in the reserve (A. Mertl-Millhollen pers. comm.). We saw several species of snakes and a small viverrid (fanaloka: Fossa fossana) there, but they do not prey upon sifakas (Goodman 2003). Because Kirindy verreauxi are likely to be exposed to a higher predation risk by terrestrial predators than Berenty verreauxi, they might associate growls predominantly with the presence of a terrestrial carnivore.
In coquereli, growls were used in similar contexts (Table I), but elicited opposite responses in the captive and wild population: Captive coquereli displayed a raptor-specific escape response, thus categorizing growls in the same way as roaring barks, and associated both alarm calls with the presence of a raptor. In contrast, wild coquereli did not seem to associate a specific threat with growls. After broadcasting this call, sifakas looked downwards, only one individual climbed up, and only a few individuals emitted growls in return. The apparent difference in meaning of growls in the 2 populations may also be due to differences in predator exposure history. Captive coquereli are exposed to red-tailed hawks and foxes. However, only attacks of red-tailed hawks have ever been observed (D. Haring pers. comm.), suggesting that they are threatened mainly by raptors. Wild coquereli at Ankarafantsika, in contrast, are exposed to raptors, viverrids, and snakes, which all hunt on them (Burney 2002; Dollar et al. 2007). Captive and wild coquereli sometimes produce growls at the end of roaring bark bouts, where they may serve principally as an indicator of arousal. This effect may have become more specific and intensified in the captive population due to a higher predation risk by aerial than terrestrial predators. In other words, because the threat of predation by raptors is far more common in the natural habitat enclosures than threats from terrestrial predators, captive coquereli may have changed the meaning of one of their alarm calls along with this shift in the predominant predation regime (Fichtel and van Schaik 2006). Thus, the potential meaning of growls seems to be most variable between populations in both species. Both verreauxi and coquereli seem to associate growls with the presence of the predator, to which the respective population is more strongly exposed to terrestrial predators for verreauxi in the habitat with a higher occurrence of fossas and dogs, and raptors for coquereli in the habitat where raptors are relatively more prevalent.
Tchi-faks
At Kirindy and Berenty, verreauxi at Kirindy and Berenty exhibited slightly different responses to playback experiments with tchi-faks. Kirindy verreauxi responded with a different alarm call (growl) and some climbed down; among Berenty verreauxi only 1 individual responded with tchi-faks, and some of them climbed up and others climbed down. Although looking directions did not differ, members of both populations exhibited different escape responses to tchi-faks. Both populations use tchi-faks and growls similarly in nonpredatory contexts (Fichtel and Kappeler 2002; Fichtel and van Schaik 2006). However, in predatory contexts, Kirindy verreauxi produced tchi-faks only when they were directly attacked and chased by fossas, whereas Berenty verreauxi produced them also in response to fanalokas and small snakes (Table I). Because large terrestrial predators are not regularly present at Berenty, they may not need to associate a consistent escape strategy with tchi-faks. The specific meaning of these calls might then be inferred from the context or the caller’s gaze direction or body posture, cues that may provide information about an individual’s focus of attention and the potential threat that elicits fear in the caller (Fichtel 2004; Partan and Marler 1999). However, in coquereli both populations used tchi-faks in the same predatory and nonpredatory contexts and responded similarly to playbacks of tchi-faks.
In summary, Propithecus verreauxi and P. coquereli in each of 4 populations did not differ in usage and comprehension of roaring barks, but in the relative usage and comprehension of growls and tchi-faks. The potential meaning of growls seems to be most variable between populations in both species, with individuals associating growls with the presence of the predator, to which the respective population is more strongly exposed. Variation in anti-predator strategies as a function of different predation regimes has also been reported in Diana monkeys (Cercopithecus dianae) and tantalus monkeys (Cercopithecus aethiops tantalus), which adjusted alarm-calling behavior according to different predation regimes (Bshary 2001; Kavanagh 1980; Stephan and Zuberbühler 2008). Thus, primates seem to be able to adjust their alarm calling behavior and also the meaning of alarm calls, as shown in this study, in response to different predation threats.
Potential Mechanisms
Variation in comprehension of alarm calls may reflect differences in either innate predispositions to produce and use calls in particular contexts or in developmentally induced determinants of behavior. If usage and comprehension of alarm calls are influenced primarily by innate predispositions, one would expect that the 2 closely related species, and certainly members of the same species from different populations, exhibited very similar behavior in this context. However, only the usage and comprehension of roaring barks appeared similar between species. Tchi-faks and growls were used in some comparable, but also in some different contexts, indicating that comprehension of these calls is more flexible. The differences in meaning of growls between the 2 populations of each species may therefore be due to differences in predator exposure history and reflect the different outcomes of developmental processes used to acquire the association of a call with its referent. In terms of an evolutionary sequence, growls, which serve as indicators of increased arousal in nonpredatory contexts, might have acquired a more specific meaning in habitats in which sifakas are exposed to either a raptor- or carnivore-dominated predation risk. How a shift in meaning of a particular call is implemented behaviorally and transmitted within the population is an intriguing question for future research in animal communication.
Theory suggests that social learning is adaptive whenever asocial learning is costly (Griffin 2004; Laland 2004). Learning to recognize predators and learning of appropriate predator evasion tactics may provide the ecologically most important examples where the costs of learning become relevant. The importance of social influences on the acquisition of anti-predator behavior, such as the social transmission of predator recognition, has also been emphasized in other species. For example, blackbirds, tammar wallabies, and rhesus monkeys can be conditioned to predators or formerly novel objects by observing conspecifics’ anti-predator behavior or mobbing responses toward these objects (Cook and Mineka 1989; Curio et al. 1978; Griffin and Evans 2003). Fish and even insects are known to respond with anti-predator behavior to chemical substances released from the damaged skin of conspecifics, and this experience facilitates learning of anti-predator behavior (Chivers and Smith 1995; Krause 1993; Magguran 1989). Thus, observation-based learning of anti-predator behavior is a common adaptive process that allows fine-tuned adaptations to the local predation regime (Fichtel in press).
Conclusions
We propose that the observed population differences in responses to tchi-faks and growls might be related to differences in the set of predators to which these sifakas are exposed. Because non-human primate’s comprehension of vocalizations is highly flexible (Seyfarth and Cheney 2010) and because the appropriate categorization of alarm calls develops with age and seems to be influenced by social learning (Fichtel 2008; Hauser 1988; Seyfarth and Cheney 1997), our data suggest that sifakas of both species underwent a socially learned modification in the meaning of one of their alarm calls. However, the social and behavioral mechanisms underlying such a shift in comprehension of alarm calls remain obscure. By providing observational and experimental evidence for interpopulational variation in 2 species, our results set the stage for further experimental studies of the underlying mechanisms used to acquire the association of a call with its referent. Studying to which extent vocal signals of nonhuman primates might be subject to social transmission and, thus, the development of traditions, may also contribute to the development of predictions about the evolution of differences in anti-predator behavior between closely related species.
References
Bshary, R. (2001). Diana monkeys, Cercopithecus diana, adjust their anti-predator response behaviour to human hunting strategies. Behavioral Ecology and Sociobioliogy, 50, 251–256.
Burney, D. A. (2002). Sifaka predation by a large boa. Folia Primatologica, 73, 144–145.
Cheney, D. L., & Seyfarth, R. M. (2007). Baboon metaphysics. Chicago: Chicago University Press.
Chivers, D. P., & Smith, J. F. (1995). Chemical recognition of risky habitats is culturally transmitted among fathead minnows, Pimephales promelas (Osteichthyes, Cyprinidae). Ethology, 99, 286–296.
Cook, M., & Mineka, S. (1989). Observational conditioning of fear to fear-relevant versus fear-irrelevant stimuli in rhesus monkeys. Journal of Abnormal Psychology, 98, 448–459.
Crockford, C., Herbinger, I., Vigilant, L., & Boesch, C. (2004). Wild chimpanzees produce group-specific calls: A case for vocal learning? Ethology, 110, 221–243.
Curio, E., Ernst, U., & Vieth, W. (1978). Cultural transmission of enemy recognition: one function of mobbing. Science, 202, 899–901.
Dollar, L., Ganzhorn, J. U., & Goodman, S. M. (2007). Primates and other prey in the seasonally variable diet of Cryptoprocta ferox in the dry deciduous forest of western Madagascar. In S. Gursky & K. A. I. Nekaris (Eds.), Primate anti-predator strategies (pp. 63–76). New York: Springer.
Fichtel, C. (in press). Predation on primates. In: Mitani, J. C., Call, J., Kappeler, P., Palombit, R. & Silk, J. (Eds.), The evolution of primate societies. Chicago: University of Chicago Press
Fichtel, C. (2004). Reciprocal recognition in sifaka (Propithecus verreauxi verreauxi) and redfronted lemur (Eulemur fulvus rufus) alarm calls. Animal Cognition, 7, 45–52.
Fichtel, C. (2008). Ontogeny of conspecific and heterospecific alarm call recognition in wild Verreaux’s sifakas (Propithecus v. verreauxi). American Journal Primatology, 70, 127–135.
Fichtel, C., & Kappeler, P. M. (2002). Anti-predator behaviour of group-living Malagasy primates: mixed evidence for a referential alarm call system. Behavioral Ecology and Sociobiology, 51, 262–275.
Fichtel, C., & van Schaik, C. P. (2006). Semantic differences in sifaka (Propithecus verreauxi) alarm calls: a reflection of genetic or cultural variants? Ethology, 112, 839–849.
Fichtel, C., Hammerschmidt, K., & Jürgens, J. (2001). On the vocal expression of emotion. A multi-parametric analysis of different states of aversion in the squirrel monkey. Behaviour, 138, 97–116.
Fragaszy, D. M., & Perry, S. (2003). The biology of traditions. Cambridge: Cambridge University Press.
Goodman, S. (2003). Predation on lemurs. In S. M. Goodman & J. P. Benstead (Eds.), The natural history of Madagascar (pp. 1221–1228). Chicago: University of Chicago Press.
Griffin, A. S. (2004). Social learning about predators: a review and prospectus. Learning and Behaviour, 32, 131–140.
Griffin, A. S., & Evans, C. S. (2003). Social learning of anti-predator behaviour in a marsupial. Animal Behaviour, 66, 485–492.
Hammerschmidt, K., Freudenstein, T., & Jürgens, U. (2001). Vocal development in squirrel monkeys. Behaviour, 138, 1179–1204.
Hauser, M. D. (1988). How infant vervet monkeys learn to recognize starling alarm calls. Behaviour, 105, 187–201.
Hawkins, C. E., & Racey, P. A. (2008). Food habits of an endangered carnivore, Cryptoprocta ferox, in the dry deciduous forests of Western Madagascar. Journal of Mammalogy, 89, 64–74.
Janik, V. M., & Slater, P. J. (2003). Traditions in mammalian and avian vocal communication. In D. M. Fragaszy & S. Perry (Eds.), The biology of traditions (pp. 213–235). Cambridge: Cambridge University Press.
Karpanty, S. M., & Goodman, S. M. (1999). Diet of the Madagascar harrier-hawk, Polyboroides radiatus, in southeastern Madagascar. Journal of Raptor Research, 33, 313–316.
Kavanagh, M. (1980). Invasion of the forest by an African savannah monkey: behavioural adaptations. Behaviour, 73, 238–260.
Krause, J. (1993). Transmission of fright reaction between different species of fish. Behaviour, 127, 37–48.
Laland, K. N. (2004). Social learning strategies. Learning and Behaviour, 32, 4–14.
Lührs, M.-L., & Dammhahn, M. (2010). An unusual case of cooperative hunting in a solitary carnivore. Journal of Ethology, 28, 379–383.
Magguran, A. E. (1989). Acquired recognition of predator odour in the European minnow (Phoxinus phoxinus). Ethology, 82, 216–223.
Marler, P., & Slabbekoorn, H. (2002). Nature’s music: The science of bird song. San Diego: Academic.
Marler, P., Evans, C. S., & Hauser, M. D. (1992). Animal signals: Motivational, referential, or both? In H. Papousek, U. Jürgens, & M. Papousek (Eds.), Nonverbal vocal communication (pp. 66–86). Cambridge: Cambridge University Press.
Partan, S., & Marler, P. (1999). Communication goes multimodal. Science, 283, 1272–1273.
Pastorini, J., Forstner, M., & Martin, R. (2001). Phylogenetic history of sifakas (Propithecus: Lemuriformes) derived from mtDNA sequences. American Journal of Primatology, 53, 1–17.
Pollick, A., & De Waal, F. (2007). Ape gestures and language evolution. PNAS, 104, 8184–8189.
Seyfarth, R. M., & Cheney, D. (1986). Vocal development in vervet monkeys. Animal Behaviour, 34, 1640–1658.
Seyfarth, R. M., & Cheney, D. L. (1997). Some general features of vocal development in nonhuman primates. In C. T. Snowdon & M. Hausberger (Eds.), Social influences on vocal development (pp. 249–273). Cambridge: Cambridge University Press.
Seyfarth, R. M., & Cheney, D. L. (2003). Signalers and receivers in animal communication. Annual Review of Psychology, 54, 145–173.
Seyfarth, R. M., & Cheney, D. L. (2010). Production, usage, and comprehension. Brain & Language, 115, 92–100.
Snowdon, C. T. (2001). Social processes in communication and cognition in callitrichid monkeys: a review. Animal Cognition, 4, 247–257.
Stephan, C., & Zuberbühler, K. (2008). Predation increases acoustic complexity in primate alarm calls. Biology Letters, 4, 641–644.
Struhsaker, T. T. (1967). Auditory communication among vervet monkeys (Cerpithecus aethiops). In S. A. Altmann (Ed.), Social communication among primates (pp. 281–384). University of Chicago Press.
van Schaik, C. P., Ancrenaz, M., Borgen, G., Galdikas, B., Knott, C. L., & Singleton, I. (2003). Orangutan cultures and the evolution of material cultures. Science, 299, 102–105.
Whiten, A., Goodall, J., McGrew, W. C., Nishida, T., Reynolds, V., Sugiyama, Y., et al. (1999). Culture in chimpanzees. Nature, 682–685.
Winter, P., Handley, P., Ploog, D., & Schott, D. (1973). Ontogeny of squirrel monkey calls under natural conditions and under acoustical isolation. Behaviour, 47, 230–239.
Acknowledgments
We thank the late Dr. Olga Ramilijaona and Dr. Daniel Rakotondravony at the University of Antananarivo, the Commission Tripartite de Direction des Eaux et Forêts, ANGAP, Monsieur DeHaulme, and the Duke Lemur Center for their authorization and support of this study. We especially thank Alison Jolly and Anne Mertl-Millhollen for their support of this study. We also thank Mamy Razafindrasamba and Remy Ampataka for assistance with playback experiments. Finally, we thank Joanna Setchell and 2 anonymous referees for very constructive comments. The experiments complied with the current laws of the country in which they were performed. C. Fichtel was supported by the Förderverein des Deutschen Primatenzentrums and the Deutsche Forschungsgemeinschaft (Fi 929/1-2).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Fichtel, C., Kappeler, P.M. Variation in the Meaning of Alarm Calls in Verreaux’s and Coquerel’s Sifakas (Propithecus verreauxi, P. coquereli). Int J Primatol 32, 346–361 (2011). https://doi.org/10.1007/s10764-010-9472-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-010-9472-9