Abstract
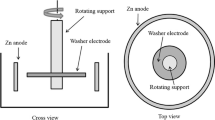
In this paper, the formation of zinc dendrites at the strip edge of an industrial electrogalvanizing line was studied. For that purpose, a rotating washer electrode was designed to reproduce the hydrodynamic conditions and the current density distribution found in the industrial process. Polarization curves were recorded at different rotation speeds in order to investigate the electrokinetic behavior of the cathodic process. The induction time for dendritic growth was estimated for different overpotential values and the existence of a minimum overpotential below which dendrites do not grow was confirmed. Dendrite precursors on the edge of the washers were well characterized and its birth and precise location were studied. The general model of disperse and dendritic metal electrodeposit formation derived by Popov et al. was used to explain the effect of electrolyte zinc concentration, rotation speed of the cathode, electrolyte temperature and edge roughness on the size and morphology of dendrites. The results showed that this theory provides an accurate description of the phenomenon even for non-stationary electrodes, which have not been extensively studied so far. The experimental setup proved to be a powerful means to study the formation of dendritic growth of zinc crystals on the edges of steel strip and is well suited to characterize other metals that produce this type of defect.










Similar content being viewed by others
References
Gamburg YD, Zangari G (2011) Theory and practice of metal electrodeposition. © Springer Science + Business Media, New York, pp 143–169. doi:10.1007/978-1-4419-9669-5
Ibl N (1983) Current distribution. In: Yeager E, Bockris IO'M, Conway BE, Sarangapani S (eds) Comprehensive treatise of electrochemistry, vol 6. Plenum Press, New York, pp 239–311
Newman JS (1991) Electrochemical systems, 2nd edn. Prentice Hall Inc., New Jersey
Plating and Electroplating (1994) In: Surface Engineering, vol 5. ASM International, ASM Handbook, p 257
Paunovic M, Schlesinger M (2006) Fundamentals of electrochemical deposition. Wiley, The Electrochemical Society Series
Banik SJ, Akolkar R (2013) Suppressing dendrite growth during zinc electrodeposition by PEG-200 Additive. J Electrochem Soc 160(11):D519–D523. doi:10.1149/2.040311jes
Kruglikov SS, Kudryavtsev NT, Vorobiova GF, Antonov AY (1965) On the mechanims of levelling by addition agents in electrodeposition of metals. Electrochim Acta 10:253–261
Bengoa LN, Bruno S, Lazzarino HA, Seré PR, Egli WA (2013) Generación de dendritas en borde de chapa durante el electrocincado en medio ácido. Paper presented at the 13er Congreso Internacional en Ciencia y Tecnología de Metalurgia y Materiales 2013, Puerto Iguazú, Argentina
Bengoa LN, Seré PR, Egli WA (2014) Influencia de la concentración de cinc en la formación de dendritas de borde en el electrocincado de chapas de acero. Paper presented at the XXI Congreso de la Sociedad Iberoamericana de Electroquímica, La Serena, Chile
Bengoa LN, Bruno S, Lazzarino HA, Seré PR, Egli WA (2014) Dendritic zinc growth on the edges of flat steel strip during electro galvanizing. Proc Mater Sci [Epub ahead of print]
Egli WA, Seré PR, Bengoa LN (2013) Formación de dendritas de cinc en electrocincado en medio ácido. Final Report, Leg. No 15282/13. Centro de Investigación y Desarrollo en Tecnologías de Pinturas (CIDEPINT)
Zubimendi JL, Baieli C, Egli W, Chara MR, Andreasen G, Schilardi P, Salvarezza R, Marchiano S (1996) The influence of operating conditions on the morphology of tin electrodeposits and tinplate quality. In: 6th Tinplate conference, London, 1996
Wranglén G (1960) Dendrites and growth layers in the electrocrystallization of metals. Electrochim Acta 2:130–146
Diggle JW, Despić AR, Bockris JOM (1969) The mechanism of the dendritic electrocrystallization of zinc. J Electrochem Soc 116(11):1503–1514
Popov KI, Maksimović MD, Trnjancev JD, Pavlović MG (1981) Dendritic electrocrystallization and the mechanism of powder formation in the potentiostatic deposition of metals. J Appl Electrochem 11(239–246):239–246
Popov KI, Djukić LM, Pavlović MG, Maksimović MD (1979) The critical overpotential for copper dendrite formation. J Appl Electrochem 9:527–531
Popov KI, Nikolic ND (2012) General theory of disperse metal electrodeposits formation. In: Djokić SS (ed) Electrochemical production of metal powders. Modern aspects of electrochemistry, vol 54. Springer Science + Business Media, New York, pp 1–62
Vetter KJ (1967) The theory of overvoltage. In: Bruckenstein S, Howard B (eds) Electrochemical kinetics. Theoretical and experimental aspects. Academic Press, New York, pp 392–395
Popov KI, Živković PM, Krstić SB, Nikolić ND (2009) Polarization curves in the ohmic controlled electrodeposition of metals. Electrochim Acta 54(10):2924–2931. doi:10.1016/j.electacta.2008.11.004
Bard AJ, Faulkner LR (2001) Electrochemical methods. Fundamental and applications, 2nd edn. Wiley, New York
Popov KI, Djokić SS, Grgur BN (2002) Surface morphology of metal electrodeposits. Fundamental aspects of electrometallurgy. Kluwer Academic/Plenum Publishers, New York, pp 29–100
Despić AR, Popov KI (1972) Transport control deposition and dissolution of metals. In: Conway BE, Bockris JOM (eds) Modern aspects of electrochemistry, vol 7. Butterworths, London, pp 304–310
Nikolić ND, Popov KI, Živković PM, Branković G (2013) A new insight into the mechanism of lead electrodeposition: ohmic-diffusion control of the electrodeposition process. J Electroanal Chem 691:66–76. doi:10.1016/j.jelechem.2012.12.011
Nikolić ND, Branković G, Lačnjevac UČ (2012) Formation of two-dimensional (2D) lead dendrites by application of different regimes of electrolysis. J Solid State Electrochem 16:2121–2126. doi:10.1007/s10008-011-1626-y
Jović VD, Nikolić ND, Lačnjevac UČ, Jović BM, Popov KI (2012) Morphology of different electrodeposited pure metal powders. In: Djokić SS (ed) Electrochemical production of metal powders. Modern aspects of electrochemistry, vol 54. Springer Science + Business Media, New York, pp 63–123
Pavlović MG, Kindlová S, Rousar I (1992) The initiation of dendritic growth of electrodeposited copper on a rotating disc electrode with changing copper concentration and diffusion layer thickness. Electrochim Acta 37(1):23–27
Gabe DR, Wilcox GD, Gonzalez-Garcia J, Walsh FC (1998) The rotating cylinder electrode: its continued development and application. J Appl Electrochem 28:759–780
Blurton KF, Riddiford AC (1965) Shapes of practical rotating disc electrodes. J Electroanal Chem 10:457–464
Levich VG (1962) Physicochemical hydrodynamics. Prentice-Hall, Englewood Cliffs
Popov KI, Pavlovic MG, Maksimovic MD (1982) Comparison of the critical conditions for initiation of dendritic growth and powder formation in potentiostatic and galvanostatic copper electrodeposition. J Appl Electrochem 12:525–531
Acknowledgments
The authors would like to thank the Comisión de Investigaciones Científicas de la Provincia de Buenos Aires (CICPBA), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Universidad Nacional de La Plata (UNLP) for their financial support to this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bengoa, L.N., Bruno, S., Lazzarino, H.A. et al. Study of dendritic growth of zinc crystals on the edges of steel sheet. J Appl Electrochem 44, 1261–1269 (2014). https://doi.org/10.1007/s10800-014-0722-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-014-0722-y