Abstract
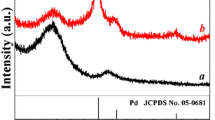
Palladium (Pd) dendrites on carbon black support were synthesized by a simple template/surfactant-free electrochemical deposition. In comparison to Pd spherical deposit obtained on non-activated carbon, Pd deposited on an electrochemically activated carbon displayed a dendritic morphology with increased electrochemical surface area and showed enhanced catalytic activity for formic acid oxidation. The effect of electrochemical activation and deposition cycles were studied in relation to the growth and morphological features of Pd deposit. Scanning electron micrographs and X-ray diffraction studies showed a transition in Pd morphology from spheres to dendrites when the carbon support was subjected to varying cycles of electrochemical activation, prior to Pd deposition. Raman and X-ray photoelectron spectra results showed that the defects induced during electrochemical activation on carbon played a major role in tailoring the Pd morphology.












Similar content being viewed by others
References
Guo S, Wang E (2011) Noble metal nanomaterials: controllable synthesis and application in fuel cells and analytical sensors. Nano Today 6:240–264. doi:10.1016/j.nantod.2011.04.007
Shao M (2011) Palladium-based electrocatalysts for hydrogen oxidation and oxygen reduction reactions. J Power Sources 196:2433–2444. doi:10.1016/j.jpowsour.2010.10.093
Durand J, Teuma E, Gómez M (2008) An overview of palladium nanocatalysts: surface and molecular reactivity. Eur J Inorg Chem 2008:3577–3586. doi:10.1002/ejic.200800569
Guo L, Searson PC (2010) On the influence of the nucleation overpotential on island growth in electrodeposition. Electrochim Acta 55:4086–4091. doi:10.1016/j.electacta.2010.02.038
Corduneanu O, Diculescu VC (2008) Shape-controlled palladium nanowires and nanoparticles electrodeposited on carbon electrodes. J Electroanal Chem 624:97–108. doi:10.1016/j.jelechem.2008.07.034
Mohanty US (2011) Electrodeposition: a versatile and inexpensive tool for the synthesis of nanoparticles, nanorods, nanowires, and nanoclusters of metals. J Appl Electrochem 41:257–270. doi:10.1007/s10800-010-0234-3
Thirumalairajan S, Girija K, Ganesh V et al (2013) Novel synthesis of LaFeO3 nanostructure dendrites: a systematic investigation of growth mechanism, properties, and biosensing for highly selective determination of neurotransmitter compounds. ACS Cryst Growth Des 13:291–302
Zhang HUI, Jin M, Xiong Y et al (2013) Shape controlled synthesis of Pd nanocrystals and their catalytic applications. Acc Chem Res 46:1783–1794. doi:10.1021/ar300209w
Patra S, Viswanath B, Barai K et al (2010) High-surface step density on dendritic Pd leads to exceptional catalytic activity for formic acid oxidation. ACS Appl Mater Interface 2:2965–2969. doi:10.1021/am100647u
Mohanty A, Garg N, Jin R (2010) A universal approach to the synthesis of noble metal nanodendrites and their catalytic properties Angew. Chem Int Ed 49:4962–4966. doi:10.1002/anie.201000902
Zheng X, Zhu L, Wang X et al (2004) A simple mixed surfactant route for the preparation of noble metal dendrites. J Cryst Growth 260:255–262. doi:10.1016/j.jcrysgro.2003.08.006
Libbrecht KG (2005) The physics of snow crystals. Rep Prog Phys 68:855–895. doi:10.1088/0034-4885/68/4/R03
Arjona N, Guerra-Balcázar M, Cuevas-Muñiz FM et al (2013) Electrochemical synthesis of flower-like Pd nanoparticles with high tolerance toward formic acid electrooxidation. RSC Adv 3:15727–15733. doi:10.1039/c3ra41681j
Zhang G, Sun S, Cai M et al (2013) Porous dendritic platinum nanotubes with extremely high activity and stability for oxygen reduction reaction. Sci Rep 3:1526. doi:10.1038/srep01526
Sharma DK, Ott A, Mullane APO, Bhargava SK (2011) The facile formation of silver dendritic structures in the absence of surfactants and their electrochemical and SERS properties. Colloids Surf A 386:98–106. doi:10.1016/j.colsurfa.2011.07.001
Zhang S, Shao Y, Yin G, Lin Y (2013) Recent progress in nanostructured electrocatalysts for PEM fuel cells. J Mater Chem A 1:4631–4641. doi:10.1039/c3ta01161e
Lin T-H, Lin C-W, Liu H-H et al (2011) Potential-controlled electrodeposition of gold dendrites in the presence of cysteine. Chem Commun 47:2044–2046. doi:10.1039/c0cc03273e
Zhou R, Zhou W, Zhang H et al (2011) Facile template-free synthesis of pine needle-like Pd micro/nano-leaves and their associated electro-catalytic activities toward oxidation of formic acid. Nanoscale Res Lett 6:381–386. doi:10.1186/1556-276X-6-381
Shim JH, Kim YS, Kang M et al (2012) Electrocatalytic activity of nanoporous Pd and Pt: effect of structural features. Phys Chem Chem Phys 14:3974–39799. doi:10.1039/c2cp23429g
Li G-R, Xu H, Lu X-F et al (2013) Electrochemical synthesis of nanostructured materials for electrochemical energy conversion and storage. Nanoscale 5:4056–4069. doi:10.1039/c3nr00607g
Xiong Y, Cai H, Wiley BJ et al (2007) Synthesis and mechanistic study of palladium nanobars and nanorods. J Am Chem Soc 129:3665–3675. doi:10.1021/ja0688023
Kangasniemi KH, Condit DA, Jarvi TD (2004) Characterization of vulcan electrochemically oxidized under simulated PEM fuel cell conditions. J Electrochem Soc 151:E125–E132. doi:10.1149/1.1649756
Lee K, Zhang J, Wang H, Wilkinson DP (2006) Progress in the synthesis of carbon nanotube- and nanofiber-supported Pt electrocatalysts for PEM fuel cell catalysis. J Appl Electrochem 36:507–522. doi:10.1007/s10800-006-9120-4
Cheng TT, Gyenge EL (2009) Novel catalyst-support interaction for direct formic acid fuel cell anodes: Pd electrodeposition on surface-modified graphite felt. J Appl Electrochem 39:1925–1938. doi:10.1007/s10800-009-9901-7
Martín AJ, Chaparro AM, Gallardo B et al (2009) Characterization and single cell testing of Pt/C electrodes prepared by electrodeposition. J Power Sources 192:14–20. doi:10.1016/j.jpowsour.2008.10.104
Maniam KK, Chetty R (2013) Electrodeposited palladium nanoflowers for electrocatalytic applications. Fuel Cells 13:1196–1204. doi:10.1002/fuce.201200162
Plyasova LM, Molina IY, Gavrilov AN et al (2006) Electrodeposited platinum revisited: tuning nanostructure via the deposition potential. Electrochim Acta 51:4477–4488. doi:10.1016/j.electacta.2005.12.027
Song Y, Kim J, Park K (2009) Synthesis of Pd dendritic nanowires by electrochemical deposition. Cryst Growth Des 9:505–507
Yu J, Fujita T, Inoue A et al (2010) Electrochemical synthesis of palladium nanostructures with controllable morphology. Nanotechnology 21:85601–85607. doi:10.1088/0957-4484/21/8/085601
Zhang H, Zhou W, Du Y et al (2010) One-step electrodeposition of platinum nanoflowers and their high efficient catalytic activity for methanol electro-oxidation. Electrochem Commun 12:882–885. doi:10.1016/j.elecom.2010.04.011
Zhang H, Jiang F, Zhou R et al (2011) Effect of deposition potential on the structure and electrocatalytic behavior of Pt micro/nanoparticles. Int J Hydrog Energy 36:15052–15059. doi:10.1016/j.ijhydene.2011.08.072
Choi S, Jeong H, Choi K-H et al (2014) Electrodeposition of triangular Pd rod nanostructures and their electrocatalytic and SERS activities. ACS Appl Mater Interfaces 6:3002–3007. doi:10.1021/am405601g
Harrison JA, Hill RPJ, Thompson J (1973) kinetics of the electrodeposition of palladium. Electroanal Chem Interfacial Electrochem 47:431–440
Soreta TR, Strutwolf J, Henry O, Sullivan CKO (2010) Electrochemical surface structuring with palladium nanoparticles for signal enhancement. Langmuir 26:12293–12299. doi:10.1021/la101398g
Jia F, Wong K, Zhang L (2009) Electrochemical synthesis of nanostructured palladium of different morphology directly on gold substrate through a cyclic deposition/dissolution route. J Phys Chem C 113:7200–7206. doi:10.1021/jp900623t
Kim B, Seo D, Lee JY et al (2010) Electrochemical deposition of Pd nanoparticles on indium-tin oxide electrodes and their catalytic properties for formic acid oxidation. Electrochem Commun 12:1442–1445. doi:10.1016/j.elecom.2010.08.004
Lee I, Chan K-Y, Phillips DL (2004) 2 dimensional dendrites and 3 dimensional growth of electrodeposited platinum nanoparticles. Jpn J Appl Phys 43:767–770. doi:10.1143/JJAP.43.767
Osorio A, Silveira I, Bueno V, Bergmann C (2008) H2SO4/HNO3/HCl—functionalization and its effect on dispersion of carbon nanotubes in aqueous media. Appl Surf Sci 255:2485–2489. doi:10.1016/j.apsusc.2008.07.144
Jawhari T, Roid A, Casado J (1995) Raman spectroscopic characterisation of some commerically available carbon black materials. Carbon 33:1561–1565
Avasarala B, Moore R, Haldar P (2010) Surface oxidation of carbon supports due to potential cycling under PEM fuel cell conditions. Electrochim Acta 55:4765–4771. doi:10.1016/j.electacta.2010.03.056
Morales-lara F, Pe MJ, Altmajer-vaz D et al (2013) Functionalization of multiwall carbon nanotubes by ozone at basic pH. Comparison with oxygen plasma and ozone in gas phase. J Phys Chem C 117:11647–11655. doi:10.1021/jp4017097
Hsieh Y, Chen J, Wu P (2011) Electrochemical degradation of nafion ionomer to functionalize carbon support for methanol electro-oxidation. J Power Sources 196:8225–8233. doi:10.1016/j.jpowsour.2011.05.068
Huang H, Wang X (2012) Pd nanoparticles supported on low-defect graphene sheets: for use as high-performance electrocatalysts for formic acid and methanol oxidation. J Mater Chem 22:22533–22541. doi:10.1039/c2jm33727d
Alcaide F, Álvarez G, Cabot PL et al (2011) Testing of carbon supported Pd–Pt electrocatalysts for methanol electrooxidation in direct methanol fuel cells. Int J Hydrog Energy 36:4432–4439. doi:10.1016/j.ijhydene.2011.01.015
Maniam KK, Muthukumar V, Chetty R (2014) Approaches towards improving the dispersion of electrodeposited palladium on carbon supports. Energy Procedia 54:281–291. doi:10.1016/j.egypro.2014.07.271
Hosseini MG, Momeni MM, Khalilpur H (2012) Synthesis and characterisation of palladium nanoparticles immobilised on TiO2 nanotubes as a new high active electrode for methanol electro-oxidation. Int J Nanosci 11:1250016–1250024. doi:10.1142/S0219581X12500160
Chai J, Li F, Bao Y, Liu S (2012) Electrochemical fabrication of multiplicate palladium hierarchical architectures and their electrocatalysis toward oxidation of formic acid. J Solid State Chem 16:1203–1210. doi:10.1007/s10008-011-1473-x
Chetty R, Scott K (2007) Characterisation of thermally deposited platinum and palladium catalysts for direct formic acid fuel cells. J New Mater Electrochem Syst 10:135–142
Choi JH, Noh SY, Han SD et al (2008) Formic acid oxidation by carbon-supported palladium catalysts in direct formic acid fuel cell. Korean J Chem Eng 25:1026–1030
Xia W, Wang Y, Bergstrasser R et al (2007) Surface characterization of oxygen-functionalized multi-walled carbon nanotubes by high-resolution X-ray photoelectron spectroscopy and temperature-programmed desorption. Appl Surf Sci 254:247–250. doi:10.1016/j.apsusc.2007.07.120
Lakshminarayanan PV, Toghiani H, Pittman CU (2004) Nitric acid oxidation of vapor grown carbon nanofibers. Carbon 42:2433–2442. doi:10.1016/j.carbon.2004.04.040
Jia F, Wong K, Du R (2009) Direct growth of highly catalytic palladium nanoplates array onto gold substrate by a template-free electrochemical route. Electrochem Commun 11:519–521. doi:10.1016/j.elecom.2008.11.054
Du C, Chen M, Wang W et al (2010) Electrodeposited PdNi2 alloy with novelly enhanced catalytic activity for electrooxidation of formic acid. Electrochem Commun 12:843–846. doi:10.1016/j.elecom.2010.03.046
Duarte MME, Taberner PM, Mayer CE (1988) Electrochemical behaviour of palladium/graphite and palladium/carbon systems. Electrochem Acta 34:499–504
Berenguer R, Marco-Lozar JP, Quijada C et al (2009) Effect of electrochemical treatments on the surface chemistry of activated carbon. Carbon 47:1018–1027. doi:10.1016/j.carbon.2008.12.022
Antolini E (2009) Carbon supports for low-temperature fuel cell catalysts. Appl Catal B Environ 88:1–24. doi:10.1016/j.apcatb.2008.09.030
Ciacchi LC, Pompe W, De Vita A (2001) Initial nucleation of platinum clusters after reduction of K2PtCl4 in aqueous solution: a first principles study. J Am Chem Soc 123:7371–7380. doi:10.1021/ja002977
Mertig M, Colombi CL, Seidel R et al (2002) DNA as a selective metallization template. Nano Lett 2:841–844. doi:10.1021/nl025612r
Xia Y, Xiong Y, Lim B, Skrabalak SE (2009) Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? Angew Chem Int Ed 48:60–103. doi:10.1002/anie.200802248
Acknowledgments
We thank the Department of Science and Technology (DST), Government of India for the financial assistance under SERC Fast Track Scheme. We also thank DST-FIST for providing the instrumentation facility to the Department of Chemical Engineering at IIT Madras.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maniam, K.K., Chetty, R. Electrochemical synthesis of palladium dendrites on carbon support and their enhanced electrocatalytic activity towards formic acid oxidation. J Appl Electrochem 45, 953–962 (2015). https://doi.org/10.1007/s10800-015-0860-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-015-0860-x