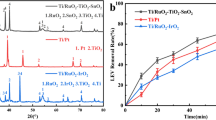
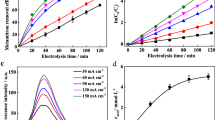
Abstract
A Ti/SnO2–Sb anode was prepared and used for the electrochemical oxidation of tetracycline (TC). The effects of the reaction conditions, including the current density (5–25 mA cm− 2), the distance between anode and cathode (5–25 mm), and the initial TC concentration (5–100 mg L− 1), on the electrochemical degradation kinetics of TC, were investigated. The degradation of TC followed pseudo-first-order kinetics (R 2 > 0.95). The TC degradation efficiency increased with the applied current density and decreased with the increase of the distance between anode and cathode. The TC degradation efficiency was almost the same when the initial TC concentration was less than 20 mg L− 1 but decreased when it was higher than 20 mg L− 1. Higher total organic carbon removal efficiency was obtained with a higher applied current density. The mineralization current efficiency gradually fell with increasing applied current density. A degradation pathway was proposed for the electrochemical oxidation of TC: Radicals produced from electrochemical oxidation attack the double bonds, phenolic group, and amine group in TC, resulting in the formation of the intermediate compounds with m/z values of 461, 477, 432, 509, 480, 448, and 525, which are then oxidized to an intermediate with m/z value of 496.
Graphical Abstract






Similar content being viewed by others
References
Wang H, Wang N, Wang B, Fang H, Fu C, Jiang F, Zhou Y, He G, Zhao Q, Chen Y, Jiang Q (2016) Antibiotics detected in urines and adipogenesis in school children. Environ Int 89:204–211
Choi YJ, Kim LH, Zoh KD (2016) Removal characteristics and mechanism of antibiotics using constructed wetlands. Ecol Eng 91:85–92
Güzel F, Sayğılı H (2015) Adsorptive efficacy analysis of novel carbonaceous sorbent derived from grape industrial processing wastes towards tetracycline in aqueous solution. J Taiwan Inst Chem E 60:236–240
Rathod M, Haldar S, Basha S (2015) Nanocrystalline cellulose for removal of tetracycline hydrochloride from water via biosorption: equilibrium, kinetic and thermodynamic studies. Ecol Eng 84:240–249
Liu M, Hou L, Yu S, Xi B, Zhao Y, Xia X (2013) MCM-41 impregnated with A zeolite precursor: synthesis, characterization and tetracycline antibiotics removal from aqueous solution. Chem Eng J 223:678–687
Pena A, Paulo M, Silva LJG, Seifrtova M, Lino CM, Solich P (2010) Tetracycline antibiotics in hospital and municipal wastewaters: a pilot study in Portugal. Anal Bioanal Chem 396:2929–2936
Wang H, Yao H, Sun P, Pei J, Li JD, Huang C (2015) Oxidation of tetracycline antibiotics induced by Fe(III) ions without light irradiation. Chemosphere 119:1255–1261
Sarmah AK, Meyer MT, Boxall ABA (2006) A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 65:725–759
Yu F, Li Y, Han S, Ma J (2016) Adsorptive removal of antibiotics from aqueous solution using carbon materials. Chemosphere 153:365–385
Rossi A, Alves VA, Da Silva LA, Oliveira MA, Assis DOS, Santos FA, De Miranda RRS (2009) Electrooxidation and inhibition of the antibacterial activity of oxytetracycline hydrochloride using a RuO2 electrode. J Appl Electrochem 39:329–337
Zhang H, Liu F, Wu X, Zhang J, Zhang D (2009) Degradation of tetracycline in aqueous medium by electrochemical method. Asia-Pac J Chem Eng 4:568–573
Brinzila CI, Pacheco MJ, Ciríaco L, Ciobanu RC, Lopes A (2012) Electrodegradation of tetracycline on BDD anode. Chem Eng J 209:54–61
Bejan D, Guinea E, Bunce NJ (2012) On the nature of the hydroxyl radicals produced at boron-doped diamond and Ebonex® anodes. Electrochim Acta 69:275–281
Lin H, Niu J, Xu J, Li Y, Pan Y (2013) Electrochemical mineralization of sulfamethoxazole by Ti/SnO2–Sb/Ce-PbO2 anode: Kinetics, reaction pathways, and energy cost evolution. Electrochim Acta 97:167–174
He X, Chai Z, Li F, Zhang C, Li D, Li J, Hu J (2013) Advanced treatment of biologically pretreated coking wastewater by electrochemical oxidation using Ti/RuO2-IrO2 electrodes. J Chem Technol Biot 88:1568–1575
Lin H, Niu J, Ding S, Zhang L (2012) Electrochemical degradation of perfluorooctanoic acid (PFOA) by Ti/SnO2–Sb, Ti/SnO2–Sb/PbO2 and Ti/SnO2–Sb/MnO2 anodes. Water Res 46:2281–2289
Wang Y, Shen C, Zhang M, Zhang B, Yu Y (2016) The electrochemical degradation of ciprofloxacin using a SnO2–Sb/Ti anode: influencing factors, reaction pathways and energy demand. Chem Eng J 296:79–89
Ganiyu SO, Oturan N, Raffy S, Cretin M, Esmilaire R, Hullebusch EV, Esposito G, Oturan MA (2016) Sub-stoichiometric titanium oxide (Ti4O7) as a suitable ceramic anode for electrooxidation of organic pollutants: a case study of kinetics, mineralization and toxicity assessment of amoxicillin. Water Res 106:171–182
Li D, Tang J, Zhou X, Li J, Sun X, Shen J, Wang L, Han W (2016) Electrochemical degradation of pyridine by Ti/SnO2–Sb tubular porous electrode. Chemosphere 149:49–56
Panizza M, Cerisola G (2009) Direct and mediated anodic oxidation of organic pollutants. Chem Rev 109:6541–6569
Li L, Goel RK (2010) Role of hydroxyl radical during electrolytic degradation of contaminants. J Hazard Mater 181:521–525
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Project No. 20907072).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhi, D., Qin, J., Zhou, H. et al. Removal of tetracycline by electrochemical oxidation using a Ti/SnO2–Sb anode: characterization, kinetics, and degradation pathway. J Appl Electrochem 47, 1313–1322 (2017). https://doi.org/10.1007/s10800-017-1125-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-017-1125-7