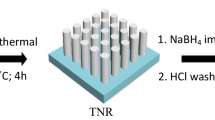
Abstract
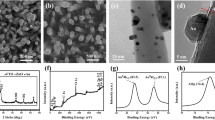
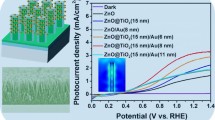
Here, the synthesis of TiO2 rutile nanorod arrays (TiO2 NRs) decorated with bimetallic gold–palladium cocatalyst nanoparticles (AuPd NPs) is described. The modified photoelectrode was characterized by field-emission scanning electron microscopy, high-resolution transmission electron microscopy, energy-dispersive spectroscopy, X-ray diffraction analysis, X-ray photoelectron spectroscopy, UV–vis spectroscopy, and electrochemical impedance spectroscopy (EIS). AuPd–TiO2 NRs (AuPd–TiO2) demonstrate high photocatalytic activity for photoelectrochemical (PEC) water splitting. The tailored structure of AuPd–TiO2 depicts a boosted photocurrent of 3.36 mA cm−2 under AM 1.5 illumination (100 mW cm−2) and efficiency of 2.31% at a low-voltage bias of 0.28 V vs. Ag–AgCl. EIS and Mott–Schottky plots reveal that AuPd–TiO2 has the lowest charge transfer resistance and highest carrier density which suggest a faster carrier transfer. These results indicate that AuPd NPs inherit both properties of light sensitizer from Au and faster electrocatalytic activity of Pd, thus not only generating hot electrons due to the surface plasmonic effect but also facilitating transfer of these electrons to the TiO2 NRs because of high electrocatalytic activity. Moreover, AuPd NPs contribute to the overall enhancement of PEC performance by producing a Schottky barrier, hindering electron–hole recombination and passivating surface defects/traps of TiO2 NRs which eventually enhances the photocurrent significantly.
Graphical Abstract










Similar content being viewed by others
References
Cook TR, Dogutan DK, Reece SY et al (2010) Solar energy supply and storage for the legacy and nonlegacy worlds. Chem Rev 110:6474–6502. https://doi.org/10.1021/cr100246c
Masudy-Panah S, Moakhar RS, Chua CS et al (2016) Rapid thermal annealing assisted stability and efficiency enhancement in a sputter deposited CuO photocathode. RSC Adv 6:29383–29390. https://doi.org/10.1039/C6RA03383K
Arani HF, Mirhabibi A (2016) Effect of nano carbon additives on the microstructure of polyvinyl chloride heated up to 2000 °C. Fullerenes Nanotubes Carbon Nanostruct 24:34–42. https://doi.org/10.1080/1536383X.2015.1110696
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37–38. https://doi.org/10.1038/238037a0
Siavash Moakhar R, Masudy-Panah S, Jalali M et al (2016) Sunlight driven photoelectrochemical light-to-electricity conversion of screen-printed surface nanostructured TiO2 decorated with plasmonic Au nanoparticles. Electrochim Acta 219:386–393. https://doi.org/10.1016/j.electacta.2016.10.022
Jalali M, Moakhar RS, Kushwaha A et al (2015) TiO2 surface nanostructuring for improved dye loading and light scattering in double-layered screen-printed dye-sensitized solar cells. J Appl Electrochem 45:831–838. https://doi.org/10.1007/s10800-015-0852-x
Yang J, Wang D, Han H, Li C (2013) Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc Chem Res 46:1900–1909. https://doi.org/10.1021/ar300227e
Park JH, Kim S, Bard AJ (2006) Novel carbon-doped TiO2 nanotube arrays with high aspect ratios for efficient solar water splitting. Nano Lett 6:24–28. https://doi.org/10.1021/nl051807y
Jalali M, Siavash Moakhar R, Kushwaha A et al (2015) Enhanced dye loading-light harvesting TiO2 photoanode with screen printed nanorod-nanoparticles assembly for highly efficient solar cell. Electrochim Acta 169:395–401. https://doi.org/10.1016/j.electacta.2015.04.077
Wang G, Yang X, Qian F et al (2010) Double-sided CdS and CdSe quantum dot co-sensitized ZnO nanowire arrays for photoelectrochemical hydrogen generation. Nano Lett 10:1088–1092. https://doi.org/10.1021/nl100250z
Siavash Moakhar R, Goh GKL, Dolati A, Ghorbani M (2017) Sunlight-driven photoelectrochemical sensor for direct determination of hexavalent chromium based on Au decorated rutile TiO2 nanorods. Appl Catal B 201:411–418. https://doi.org/10.1016/j.apcatb.2016.08.026
Siavash Moakhar R, Goh GKL, Dolati A, Ghorbani M (2015) A novel screen-printed TiO2 photoelectrochemical sensor for direct determination and reduction of hexavalent chromium. Electrochem Commun 61:110–113. https://doi.org/10.1016/j.elecom.2015.10.011
Zhang N, Liu S, Fu X, Xu YJ (2011) Synthesis of M@TiO2 (M = Au, Pd, Pt) core-shell nanocomposites with tunable photoreactivity. J Phys Chem C 115:9136–9145. https://doi.org/10.1021/jp2009989
Ran J, Zhang J, Yu J et al (2014) Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem Soc Rev 43:7787–7812. https://doi.org/10.1039/c3cs60425j
Pu YC, Wang G, Chang K, Der et al (2013) Au nanostructure-decorated TiO2 nanowires exhibiting photoactivity across entire UV-visible region for photoelectrochemical water splitting. Nano Lett 13:3817–3823. https://doi.org/10.1021/nl4018385
Takai A, Kamat PV (2011) Capture, store, and discharge. Shuttling photogenerated electrons across TiO2-silver interface. ACS Nano 5:7369–7376. https://doi.org/10.1021/nn202294b
Amirav L, Alivisatos AP (2010) Photocatalytic hydrogen production with tunable nanorod heterostructures. J Phys Chem Lett 1:1051–1054. https://doi.org/10.1021/jz100075c
Zhang Z, Yu Y, Wang P (2012) Hierarchical top-porous/bottom-tubular TiO2 nanostructures decorated with Pd nanoparticles for efficient photoelectrocatalytic decomposition of synergistic pollutants. ACS Appl Mater Interfaces 4:990–996. https://doi.org/10.1021/am201630s
Moakhar RS, Kushwaha A, Jalali M et al (2016) Enhancement in solar driven water splitting by Au–Pd nanoparticle decoration of electrochemically grown ZnO nanorods. J Appl Electrochem 46:819–827. https://doi.org/10.1007/s10800-016-0981-x
Su F, Wang T, Lv R et al (2013) Dendritic Au/TiO2 nanorod arrays for visible-light driven photoelectrochemical water splitting. Nanoscale 5:9001–9009. https://doi.org/10.1039/c3nr02766j
Zhou X, Liu G, Yu J, Fan W (2012) Surface plasmon resonance-mediated photocatalysis by noble metal-based composites under visible light. J Mater Chem 22:21337. https://doi.org/10.1039/c2jm31902k
Lee J, Mubeen S, Ji X et al (2012) Plasmonic photoanodes for solar water splitting with visible light. Nano Lett 12:5014–5019
Pu YC, Chen YC, Hsu YJ (2010) Au-decorated NaxH2-xTi3O7 nanobelts exhibiting remarkable photocatalytic properties under visible-light illumination. Appl Catal B 97:389–397
Zhang Z, Zhang L, Hedhili MN et al (2013) Plasmonic gold nanocrystals coupled with photonic crystal seamlessly on TiO2 nanotube photoelectrodes for efficient visible light photoelectrochemical water splitting. Nano Lett. https://doi.org/10.1021/Nl3029202
Wang Y, Chu W, Wang S et al (2014) Simple synthesis and photoelectrochemical characterizations of polythiophene/Pd /TiO2 composite microspheres. ACS Appl Mater Interfaces 6:20197–20204
Panigrahy B, Sarma DD (2014) Enhanced photocatalytic efficiency of AuPd nanoalloy decorated ZnO-reduced graphene oxide nanocomposites. RSC Adv 5:8918–8928. https://doi.org/10.1039/C4RA13245A
Masudy-Panah S, Siavash Moakhar R, Chua CS et al (2017) Stable and efficient CuO based photocathode through oxygen-rich composition and Au–Pd nanostructure incorporation for solar-hydrogen production. ACS Appl Mater Interfaces 9:27596–27606. https://doi.org/10.1021/acsami.7b02685
Zolriasatein A, Shokuhfar A, Safari F, Abdi N (2017) Comparative study of SPEX and planetary milling methods for the fabrication of complex metallic alloy nanoparticles. Micro Nano Lett 13:448–451. https://doi.org/10.1049/mnl.2017.0608
Zolriasatein A, Shokuhfar A (2015) Size effect on the melting temperature depression of Al12Mg17 complex metallic alloy nanoparticles prepared by planetary ball milling. Phys E 74:101–107. https://doi.org/10.1016/j.physe.2015.06.015
Hou W, Dehm N, Scott RWJ (2008) Alcohol oxidations in aqueous solutions using Au, Pd, and bimetallic AuPd nanoparticle catalysts. J Catal 253:22–27. https://doi.org/10.1016/j.jcat.2007.10.025
Liu B, Aydil ES (2009) Growth of oriented single-crystalline rutile TiO2 nanorods on transparent conducting substrates for dye-sensitized solar cells. J Am Chem Soc 131:3985–3990. https://doi.org/10.1021/ja8078972
Rutile phase, Joint Committee on Powder Diffraction Standards (JCPDC) card no 21-1276
Gold, Joint Committee on Powder Diffraction Standards (JCPDC) card no 04-0784
Palladium, Joint Committee on Powder Diffraction Standards (JCPDC) card no 01-1201
Kruse N, Chenakin S (2011) XPS characterization of Au/TiO2 catalysts: binding energy assessment and irradiation effects. Appl Catal A 391:367–376. https://doi.org/10.1016/j.apcata.2010.05.039
Fang J, Cao SW, Wang Z et al (2012) Mesoporous plasmonic Au–TiO2 nanocomposites for efficient visible-light-driven photocatalytic water reduction. Int J Hydrogen Energy 37:17853–17861. https://doi.org/10.1016/j.ijhydene.2012.09.023
Lay B, Sabri YM, Ippolito SJ, Bhargava SK (2014) Galvanically replaced Au–Pd nanostructures: study of their enhanced elemental mercury sorption capacity over gold. Phys Chem Chem Phys 16:19522–19529. https://doi.org/10.1039/c4cp02233e
Brun M, Berthet A, Bertolini J (1999) XPS, AES and Auger parameter of Pd and PdO. J Electron Spectrosc Relat Phenom 104:55–60. https://doi.org/10.1016/S0368-2048(98)00312-0
Liu Y, Juang L (2004) Electrochemical methods for the preparation of gold-coated TiO2 nanoparticles with variable coverages. Langmuir 20:6951–6955
Wu Y, Liu H, Zhang J, Chen F (2009) Enhanced photocatalytic activity of nitrogen-doped titania by deposited with gold. J Phys Chem C 113:14689–14695. https://doi.org/10.1021/jp904465d
Zielińska-Jurek A, Kowalska E, Sobczak JW et al (2011) Preparation and characterization of monometallic (Au) and bimetallic (Ag/Au) modified-titania photocatalysts activated by visible light. Appl Catal B 101:504–514. https://doi.org/10.1016/j.apcatb.2010.10.022
Pal A, Shah S, Devi S (2007) Preparation of silver, gold and silver-gold bimetallic nanoparticles in w/o microemulsion containing TritonX-100. Colloids Surf A 302:483–487. https://doi.org/10.1016/j.colsurfa.2007.03.032
Ferrando R, Jellinek J, Johnston RL (2008) Nanoalloys: from theory to applications of alloy clusters and nanoparticles. Chem Rev 108:845–910. https://doi.org/10.1021/cr040090g
Maringa A, Mashazi P, Nyokong T (2015) Electrocatalytic activity of bimetallic Au-Pd nanoparticles in the presence of cobalt tetraaminophthalocyanine. J Colloid Interface Sci 440:151–161. https://doi.org/10.1016/j.jcis.2014.10.056
Parkinson B (1984) On the efficiency and stability of photoelectrochemical devices. Acc Chem Res 17:431–437. https://doi.org/10.1021/ar00108a004
Zielińska-Jurek A (2014) Progress, challenge, and perspective of bimetallic TiO2-based photocatalysts. J Nanomater 2014:1–17. https://doi.org/10.1155/2014/208920
Mahshid S, Li C, Mahshid SS et al (2011) Sensitive determination of dopamine in the presence of uric acid and ascorbic acid using TiO2 nanotubes modified with Pd, Pt and Au nanoparticles. Analyst 136:2322–2329. https://doi.org/10.1039/c1an15021a
Dimitratos N, Porta F, Prati L (2005) Au, Pd (mono and bimetallic) catalysts supported on graphite using the immobilisation method: synthesis and catalytic testing for liquid phase oxidation of glycerol. Appl Catal A 291:210–214. https://doi.org/10.1016/j.apcata.2005.01.044
Gomes Silva C, Juárez R, Marino T et al (2011) Influence of excitation wavelength (UV or visible light) on the photocatalytic activity of titania containing gold nanoparticles for the generation of hydrogen or oxygen from water. J Am Chem Soc 133:595–602. https://doi.org/10.1021/ja1086358
Balcha T, Strobl JR, Fowler C et al (2011) Selective aerobic oxidation of crotyl alcohol using aupd core-shell nanoparticles. ACS Catal 1:425–436. https://doi.org/10.1021/cs200040a
Dhara S, Giri PK (2011) On the origin of enhanced photoconduction and photoluminescence from Au and Ti nanoparticles decorated aligned ZnO nanowire heterostructures. J Appl Phys 110:124317. https://doi.org/10.1063/1.3671023
Wei C, Bee S, Basirun WJ (2014) Effect of Ce doping on RGO-TiO2 nanocomposite for high photoelctrocatalytic behavior. Int J Photoenergy 2014:1–9. https://doi.org/10.1155/2014/141368
Dupuy L, Haller S, Rousset J et al (2010) Impedance measurements of nanoporosity in electrodeposited ZnO films for DSSC. Electrochem Commun 12:697–699. https://doi.org/10.1016/j.elecom.2010.03.009
Guo X, Diao P, Xu D et al (2014) CuO/Pd composite photocathodes for photoelectrochemical hydrogen evolution reaction. Int J Hydrogen Energy 39:7686–7696. https://doi.org/10.1016/j.ijhydene.2014.03.084
Parker RA (1961) Static dielectric constant for rutile (TiO2), 1.6-1060 K. Phys Rev 124:1719–1722
Wang Y, Zhang YY, Tang J et al (2013) Simultaneous etching and doping of TiO2 nanowire arrays for enhanced photoelectrochemical performance. ACS Nano 7:9375–9383. https://doi.org/10.1021/nn4040876
Hou W, Cronin SB (2013) A review of surface plasmon resonance-enhanced photocatalysis. Adv Funct Mater 23:1612–1619. https://doi.org/10.1002/adfm.201202148
Haro M, Abargues R, Herraiz-Cardona I et al (2014) Plasmonic versus catalytic effect of gold nanoparticles on mesoporous TiO2 electrodes for water splitting. Electrochim Acta 144:64–70. https://doi.org/10.1016/j.electacta.2014.07.146
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Siavash Moakhar, R., Jalali, M., Kushwaha, A. et al. AuPd bimetallic nanoparticle decorated TiO2 rutile nanorod arrays for enhanced photoelectrochemical water splitting. J Appl Electrochem 48, 995–1007 (2018). https://doi.org/10.1007/s10800-018-1231-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-018-1231-1