Abstract
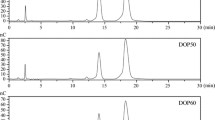
Polysaccharides extracted from Ulva pertusa Kjellm (Chlorophyta) are a group of sulfated heteropolysaccharides, the ulvans. In this study, different molecular weight ulvans were prepared by H2O2 degradation and their antioxidant activities investigated including superoxide and hydroxyl radical scavenging activity, reducing power and metal chelating ability. The molecular weights of natural and degraded ulvans were 151.7, 64.5, 58.0, and 28.2 kDa, respectively, as determined by high performance gel permeation chromatography. Among the four samples, U3 (the lowest molecular weight sample) showed significant inhibitory effects on superoxide and hydroxyl radicals with IC50 values of 22.1 μ g mL−1 and 2.8 mg mL−1; its reducing power and metal chelating ability were also the strongest among the four samples. All the other samples also demonstrated strong activity against superoxide radicals. The results indicated that molecular weight had a significant effect on the antioxidant activity of ulvan with low molecular weight ulvan having stronger antioxidant activity.
Similar content being viewed by others
Abbreviations
- U:
-
polysaccharide extracted from U. pertusa (ulvan, Mw, 151.7 kDa)
- U1 :
-
degraded ulvan of molecular weight 64.5 kDa
- U2 :
-
degraded ulvan of molecular weight 58.0 kDa
- U3 :
-
degraded ulvan of molecular weight 28.2 kDa
- NBT:
-
nitro blue tetrazolium
- PMS:
-
phenazine methosulfate
- NADH:
-
nicotinamide adenine dinucleotide-reduced
- EDTA:
-
ethylene diamine tetra-acetic acid
- TCA:
-
trichloroacetic acid
- TBA:
-
thiobarbituric acid
- DR:
-
deoxyribose
- HPGPC:
-
high performance gel permeation chromatography
- GC:
-
gas liquid chromatography
References
Chen H, Wang K (1997) Function and structure of glycoconjugates. Shanghai University Press, Shanghai, China, 341pp. (in Chinese).
Chen HX, Zhang M, Xie BJ (2005) Components and antioxidant activity of polysaccharide conjugate from green tea. Food Chem. 90: 17–21.
Cheng S, Wang JW, Fang L, Gao XD, Tan RX (2004) Free radical scavenging and antioxidant activities of EPS2, an exopolysaccharide produced by a marine filamentous fungus Keissleriella sp. YS 4108. Life Sci. 75: 1063–1073.
Colliec S, Boisson-Vidal C, Jozefonvicz J (1994) A low molecular weight fucoidan fraction from the brown seaweed Pelvetia canaliculata. Phytochem. 35: 697–700.
Council of Chinese Pharmacopoeia (1995) Chinese Pharmacopoeia. Chemistry Industry Press, Beijing, China. 36pp (in Chinese)
Dahl MK, Richardson T (1978) Photogeneration of superoxide anion in serum of bovine milk and in model systems containing riboflavin and amino acids. J. Dairy Sci. 61: 400-407.
Ferial HB, Mosstafa E, Corinne S, Catherine BV (2000) Relationship between sulfate group and biological activities of fucans. Thromb. Res. 100: 453–459.
Gordon MH (1990) The Mechanism of Antioxidant Action in vitro. In: Hudson BJF (ed.), Food Antioxiants. Elsevier Applied Science, London and New York. pp. 1–18.
Halliwell B, Gutteridge JMC, Aruoma OI (1987) The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem. 165: 215–219.
Halliwell B, Gutteridge JM, Cross CE (1992) Free radicals, antioxidants, and human disease: Where are we now? J. Lab. Clin. Med. 119: 598–620.
Hettiarachchy NS, Glenn KC, Ghanasambandan R, Johnson MG (1996) Natural antioxidant extract from fenugreek (Trigonella foenumgraecum) for ground beef patties. J. Food Sci. 61: 516–519.
Hultin HO (1992) Lipid Oxidation in Muscle Foods via Redox Iron. In Angelo AJ (ed.) Lipid oxidation in food. American Chemical Society, Washington. pp. 105–113.
Kanner J (1990) Nitric oxide, a super antioxidant. Free Radical Biol. Med. 9 (Suppl): 15.
Kawai Y, Seno N, Anno K (1969) A modified method for chondrosulfatase assay. Anal. Biochem. 32: 314–321.
Lahaye M (1998) NMR spectroscopic characterization of oligosaccharides from two Ulva rigida ulvan samples (Ulvales, Chlorophyta) deraded by a lyase. Carbohydr. Res. 314: 1–12.
Lahaye M, Jegon D (1993) Chemical and physical-chemical characteristics of dietary fibers from Ulva lactuca (L.) Thuret and Enteromorpha compressa (L.) Grev. J. Appl. Phycol. 5: 195-200.
Lahaye M, Alvarez-Cable Cimadevilla E, Kuhlenkamp R, Quemener B, Lognoné V, Dion P (1999) Chemical composition and 13C-NMR spectroscopic characterisation of ulvans from Ulva(Ulvales, Chlorophyta). J. Appl. Phycol. 11: 1–7.
Lopes GKB, Schulman HM, Hermeslima M (1999) Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton ions. Biochim. Biophys. Acta. 1472: 142–152.
Luo Q, Cai YZ, Yan J, Sun M, Corke H (2004) Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 76: 137–149.
Nishimiki M, Rao NA, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 46: 849–853.
Park PJ, Jung WK, Nam KS, Shahidi F, Kim SK (2001) Purification and characterization of antioxidative peptides from protein hydrolysate of lecithin-free egg yolk. J. Am. Oil Chem. Soc. 78: 651–656.
Pereira MS, Mulloy B, Mourao PAS (1999) Structure and anticoagulant activity of sulfated fucans. Comparison between the regular, repetitive, and linear fucans from echinoderms with the more heterogeneous and branched polymers from brown algae. J. Biol. Chem. 274: 7656–7667.
Pin-Der D, Pin-Chan D, Gow-Chin Y (1999) Action of methanolic extract of mung bean hulls as inhibitors of lipid peroxidation and non-lipid oxidative damage. Food Chem. Toxicol. 37: 1055–1061.
Quemener B, Lahaye M, Bobin-Dubigeon C (1997) Sugar determination in ulvans by a chemical-enzymatic method coupled to high performance anion exchange chromatography. J. Appl. Phycol. 9: 179–188.
Ray B, Lahaye M (1995) Cell-wall polysaccharide from the marine green alga Ulva “rigida” (Ulvales, Chlorophyta). Extraction and chemical composition. Carbohydr. Res. 274: 251-261.
Shon MY, Kim TH, Sung NJ (2003) Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetaceae) extracts. Food Chem. 82: 593-597.
Smith C, Halliwell B, Aruoma OI (1992) Protection by albumin against the pro-oxidant actions of phenolic dietary components. Food Chem. Toxicol. 30: 483–489.
Thibault JF (1979) Automatisation du dosage des substances pectiques par la méthode au méta-hydroxydiphényl. Lebensm. Wiss. Technol. 12: 247–251.
Tsiapali E, Whaley S, Kalbfleisch J, Ensley HE, Browder IW, Williams DL (2001) Glucans exhibit weak antioxidant activity, but stimulate macrophage free radical activity. Free Radicals Biol. Med. 30: 393–402.
Wanita A, Loren L (1996) Antioxidant potential of 5-N-pentadecylresorcinol. J. Food Process. Pres. 20: 417-429.
Xing RE, Yu HH, Liu S, Zhang WW, Zhang QB, Li ZE, Li PC (2005) Antioxidant activity of differently regioselective chitosan sulfates in vitro. Bioorg. Med. Chem. 13: 1387–1392.
Yamaguchi T, Takamura H, Matoba T, Terao J (1998) HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-Diphenyl-2-picrylhydrazyl. Biosci. Biotechnol. Biochem. 62: 1201–1204.
Yu PZ, L N, Liu XG, Zhou GF, Zhang QB, Li PC (2003a) Antihyperlipidemic effects of different molecular weight sulfated polysaccharides from Ulva pertusa (Chlorophyta). Pharmacol. Res. 48: 543–549.
Yu PZ, Zhang QB, Li N, Xu ZH, Wang YM, Li ZE (2003b) Polysaccharides from Ulva pertusa (Chlorophyta) and preliminary studies on their antihyperlipidemia activity. J. Appl. Phycol. 15: 21–27.
Zhang QB, Yu PZ, Li ZE, Zhang H, Xu Z, Li PC (2003) Antioxidant activities of sulfated polysaccharide fractions from Porphyra haitanesis. J. Appl. Phycol. 15: 305–310.
Zhang WJ (2003) Biochemical Technique of Glycoconjugates. Zhejiang University Press, Hangzhou, China. 38pp. (in Chinese)
Zhou GF, Sun YP, Xin H, Zhang YN, Li ZE, Xu ZH (2004) In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol. Res. 50: 47–53.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qi, H., Zhao, T., Zhang, Q. et al. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta). J Appl Phycol 17, 527–534 (2005). https://doi.org/10.1007/s10811-005-9003-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-005-9003-9