Abstract
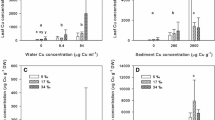
We investigated physiological responses of Ulva australis to metals and their implications for biomonitoring and management tool. To determine the capacity of Ulva to accumulate metals over the short-term, we undertook an in situ experiment where we transplanted thalli to sites with different levels of metal pollution. After 12 days, arsenic, copper, lead, and zinc accumulation was observed. Zinc was significantly greater (p = 0.001) at the most polluted site and was highly correlated (r = 0.87) with seawater total Zn concentration. We also assessed whether metal exposure can compromise U. australis performance by evaluating physiological responses and changes in thalli ultrastructure. We observed an increase in electron-dense bodies in the cell walls and vacuoles, which clearly indicates metal accumulation. However, there was no change in physiological performance (i.e. growth rate, Fv/Fm, rETRmax, or in photosynthetic pigments content) between the control and transplanted thalli (p > 0.05). Bioaccumulation capacity of U. australis was assessed by deploying thalli at a highly polluted site for 45 days, where zinc in Ulva markedly increased over time and was highly correlated with the environmental concentrations (total Zn in seawater, r = 0.85). The metal uptake rate increased steadily over time, confirming that Ulva is clearly capable of bioaccumulation. However, visual examination of the thalli suggested degradation over time, which might limit deployment time (20 days). Clearly, U. australis has potential as a biomonitor/management tool, particularly for zinc, but the results suggest it may be a useful tool for removing metals from the environment.








Similar content being viewed by others
References
Amado Filho GM, Andrade LR, Karez CS, Farina M, Pfeiffer WC (1999) Brown algae species as biomonitors of Zn and Cd at Sepetiba Bay, Rio de Janeiro, Brazil. Mar Environ Res 48:212–224
Andrade LR, Farina M, Amado Filho GM (2004) Effects of copper on Enteromorpha flexuosa (Chlorophyta) in vitro. Ecotox Environ Safe 58:117–125
ANZECC (2000) Australian and New Zealand guidelines for fresh and marine water quality, vol 1. The guidelines. Australian and New Zealand Environment and Conservation Council and the Agriculture and Resource Management Council of Australia and New Zealand
Baumann HA, Morrison L, Stengel DB (2009) Metal accumulation and toxicity measured by PAM-chlorophyll fluorescence in seven species of marine macroalgae. Ecotox Environ Safe 72:1063–1075
Bird K, Bourdine K, Busch W (1998) Marine algae as a tool for bioremediation of marine ecosystems. In: Altman A (ed) Agricultural Biotechnology. CRC Press, Boca Raton, pp 601–613
Boubonari T, Malea P, Kevrekidis T (2008) The green seaweed Ulva rigida as a bioindicator of metals (Zn, Cu, Pb and Cd) in a low-salinity coastal environment. Bot Mar 51:472–484
Bouzon ZL, Schmidt EC, Almeida AC, Yokoya NS, Oliveira MC, Chow F (2011) Cytochemical characterization and ultrastructural organization in calluses of the agarophyte Gracilariopsis tenuifrons (Gracilariales, Rhodophyta). Micron 42:80–86
Brown MT, Hodgkinson WM, Hurd CL (1999) Spatial and temporal variations in the copper and zinc concentrations of two green seaweeds from Otago Habour, New Zealand. Mar Environ Res 47:175–184
Brown MT, Newman JE, Han T (2012) Inter-population comparisons of copper resistance and accumulation in the red seaweed, Gracilariopsis longissima. Ecotoxicology 21:591–600
Buschmann AH, Cabello F, Young K, Carvajal J, Varela DA, Henríquez L (2009) Salmon aquaculture and coastal ecosystem health in Chile: analysis of regulations, environmental impacts and bioremediation systems. Ocean Coast Manage 52:243–249
Chopin T, Yarish C, Wilkes R, Belyea E, Lu S, Mathieson A (1999) Developing Porphyra/salmon integrated aquaculture for bioremediation and diversification of the aquaculture industry. J Appl Phycol 11:463–472
Conti ME, Cecchetti G (2003) A biomonitoring study: trace metals in algae and molluscs from Tyrrhenian coastal areas. Environ Res 93:99–112
Costa GB, de Felix MR, Simioni C, Ramlov F, Oliveira ER, Pereira DT, Maraschin M, Chow F, Horta PA, Lalau CM, da Costa CH, Matias WG, Bouzon ZL, Schmidt EC (2015) Effects of copper and lead exposure on the ecophysiology of the brown seaweed Sargassum cymosum. Protoplasma 253:111–125
Coughanowr C, Whitehead S, Whitehead J, Einoder L, Taylor U (2015) State of Derwent: a review of environmental data from 2009 to 2014. pp 250
El-Naggar AH, Sheikh HM (2014) Response of the green microalga Chlorella vulgaris to the oxidative stress caused by some heavy metals. Life Sci J 11:1249–1257
Felix MR, Osorio LK, Ouriques LC, Farias-Soares FL, Steiner N, Kreusch M, Pereira DT, Simioni C, Costa GB, Horta PA, Chow F, Ramlov F, Maraschin M, Bouzon ZL, Schmidt EC (2014) The effect of cadmium under different salinity conditions on the cellular architecture and metabolism in the red alga Pterocladiella capillacea (Rhodophyta, Gelidiales). Microsc Microanal 20:1411–1421
FSANZ (2006) Safe seafood Australia, a guide to the Australian primary production and processing standard for seafood, 2nd edn. Food Standards Australia New Zealand, Canberra, p 130
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92:407–418
Gledhill M, Brown MT, Nimmo M, Moate R, Hill SJ (1998) Comparision of techniques for the removal of particulate material from seaweed tissue. Mar Environ Res 45:295–307
Gouveia C, Kreusch M, Schmidt EC, Felix MR, Osorio LK, Pereira DT, dos Santos R, Ouriques LC, de Paula MR, Latini A, Ramlov F, Carvalho TJ, Chow F, Maraschin M, Bouzon ZL (2013) The effects of lead and copper on the cellular architecture and metabolism of the red alga Gracilaria domingensis. Microsc Microanal 19:513–524
Grote B (2016) Bioremediation of aquaculture wastewater: evaluating the prospects of the red alga Palmaria palmata (Rhodophyta) for nitrogen uptake. J Appl Phycol 28:3075–3082
Guisti L (2001) Heavy metal contamination of brown seaweed and sediments from the UK coastline between the wear river and the tees river. Environ Int 26:275–186
Han T, Kang SH, Park JS, Lee HK, Brown MT (2008) Physiological responses of Ulva pertusa and U. armoricana to copper exposure. Aquat Toxicol 86:176–184
Ho Y (1984) Zn and Cu concentrations in Ascophullum nodosum and Fucus vesiculosus (Phaeophyta, Fucales) after transplantation to an estuary contaminated with mine wastes. Conserv Recycling 7:329–337
Ho Y (1990) Ulva lactuca as bioindicator of metal contamination in intertidal waters in Hong Kong. Hydrobiologia 203:73–81
Huang X, Ke C, Wang W-X (2008) Bioaccumulation of silver, cadmium and mercury in the abalone Haliotis diversicolor from water and food sources. Aquaculture 283:194–202
Hurd CL, Harrison PJ, Bischof K, Lobban CS (2014) Seaweed ecology and physiology, 2nd edn. Cambridge University Press, Cambridge, p 562
Juwarkar AA, Singh SK, Mudhoo A (2010) A comprehensive overview of elements in bioremediation. Rev Environ Sci Biol 9:215–288
Kumar A, Bisht BS, Joshi VD, Dhewa T (2011) Bioremediation of polluted environment: a management tool. Int J Env Sci 1:1079–1093
Lacroix C, Richard G, Seguineau C, Guyomarch J, Moraga D, Auffret M (2015) Active and passive biomonitoring suggest metabolic adaptation in blue mussels (Mytilus spp.) chronically exposed to a moderate contamination in Brest harbor (France). Aquat Toxicol 162:126–137
Lanubile R, Piro G, Dalessandro G (1997) Effects of Brefeldin a on the synthesis and transport of cell wall polysaccharides and proteins in pea root seedling. J Exp Bot 48:1925–1933
Lee WY, Wang WX (2001) Metal accumulation in the green macroalga Ulva fasciata: effect of nitrate, ammonium and phosphate. Sci Total Environ 278:11–22
Longstaff BJ, Kildea T, Runcie JW, Cheshire A, Dennison W, Hurd CL, Kana T, Raven JA, Larkum AWD (2002) An in situ study of photosynthetic oxygen exchange and electron transport rate in the marine macroalga Ulva lactuca (Chlorophyta). Photosynth Res 74:281–293
Luoma SN, Bryan GW, W. J. L (1982) Scavenging of heavy metals from particulates by brown seaweed. Mar Pollut Bull 13:394–396
Malea P, Haritonidis S (2000) Use of the green alga Ulva rigida C. Agardh as an indicator species to reassess metal pollution in the Thermaikos Gulf, Greece, after 13 years. J Appl Phycol 12:169–176
Malea P, Haritonidis S, Kevrekidis T (1995) Metal content of some green and brown seaweeds from Antikyra Gulf (Greece). Hydrobiologia 310:19–31
Mamboya F, Lyimo TJ, Landberg T, Björk M (2009) Influence of combined changes in salinity and copper modulation on growth and copper uptake in the tropical green macroalga Ulva reticulata. Estuar Coast Shelf Sci 84:326–330
Mehta SK, Gaur JP (2005) Use of algae for removing heavy metal ions from wastewater: progress and prospects. Crit Rev Biotech 25:113–152
Munda IM (1984) Salinity dependent accumulation of Zn, Co and Mn in Scytosiphon lomentaria (Lyngb.) link and Enteromorpha intestinalis (L.) link from the Adriatic Sea. Bot Mar 27:371–376
Myklestad S, Eide I (1978) Exchange of heavy metals in Ascophyllum nodosum (L.) Le Jol. in situ by means of transplanting experiments. Environ Pollut 16:277–284
Oliveira RC, Palmieri MC, Garcia OJ (2011) Biosorption of metals: state of the art, general features, and potential applications for environmental and technological processes. In: Shaukat SS (ed) Progress in Biomas and Bionergy Production. InTech, Riejeka p 445.
PAM-Processor (2015) https://github.com/RobTheOceanographer/pam_in_R/releases. Accessed June 2015
Pellegrini L, Pellegrini M, Delivopoulos S, Berail G (1991) The effects of cadmium on the fine structure of the brown alga Cystoseira barbata forma repens Zinova et Kalugina. Brit Phycol J 26:1–8
Phillips D, Rainbow P (1994) Biomonitoring of trace aquatic contaminants. Chapman & Hall, London, p 371
Pinto E, Sigaud-Kutner T, Leitão M, Okamoto O, Morse D, Colepicolo P (2003) Heavy metal-induce oxidative stress in algae. J Phycol 39:1008–1018
Platt T, Harrison WG, Irwin B, Horne EP, Gallegos CL (1980) Photosynthesis and photoadaptation of marine phytoplankton in the Arctic. Deep Sea Res 29:1159–1170
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R-project.org/
Rainbow PS (1995) Biomonitoring of heavy metal availability in the marine environment. Mar Pollut Bull 31:183–192
Rainbow PS, Phillips DJH (1993) Cosmopolitan biomonitors of trace metals. Mar Pollut Bull 26:593–601
Ralph PJ, Gademann R (2005) Rapid light curves: a powerful tool to assess photosynthetic activity. Aquat Bot 82:222–237
Ronci L, Meccoli L, Iannilli V, Menegoni P, De Matthaeis E, Setini A (2016) Comparison between active and passive biomonitoring strategies for the assessment of genotoxicity and metal bioaccumulation in Echinogammarus veneris (Crustacea: Amphipoda). Ital J Zool 83:162–172
Ryan S, McLoughlin P, O’Donovan O (2012) A comprehensive study of metal distribution in three main classes of seaweed. Environ Pollut 167:171–177
Santos RW, Schmidt EC, Felix MR, Polo LK, Kreusch M, Pereira DT, Costa GB, Simioni C, Chow F, Ramlov F, Maraschin M, Bouzon ZL (2014) Bioabsorption of cadmium, copper and lead by the red macroalga Gelidium floridanum: physiological responses and ultrastructure features. Ecotox Environ Safe 105:80–89
Scherner F, Bonomi Barufi J, Horta PA (2012) Photosynthetic response of two seaweed species along an urban pollution gradient: evidence of selection of pollution-tolerant species. Mar Pollut Bull 64:2380–2390
Scherner F, Ventura R, Barufi JB, Horta PA (2013) Salinity critical threshold values for photosynthesis of two cosmopolitan seaweed species: providing baselines for potential shifts on seaweed assemblages. Mar Environ Res 91:14–25
Schiavon M, Moro I, Pilon-Smits EA, Matozzo V, Malagoli M, Dalla Vecchia F (2012) Accumulation of selenium in Ulva sp. and effects on morphology, ultrastructure and antioxidant enzymes and metabolites. Aquat Toxicol 122-123:222–231
Schreiber U, Klughammer C, Kolbowski J (2011) High-end chlorophyll fluorescence analysis with the MULTI-COLOR-PAM. I. Various light qualities and their applications. PAM Appl Notes 1:1–21
Troell M, Halling C, Nilsson A, Buschmann AH, Kautsky N, Kautsky L (1997) Integrated marine cultivation of Gracilaria chilensis (Gracilariales, Rhodophyta) and salmon cages for reduced environmental impact and increased economic output. Aquaculture 156:45–61
Troell M, Ronnback P, Halling C, Kaustsky N, Buschmann A (1999) Ecological engineering in aquaculture: use of seaweeds for removing nutrients from intensive mariculture. J Appl Phycol 11:89–97
Vecchia FD, Marzocchi M, Maistro S, Moro I (2012) Morpho-physiological effects of cadmium on two Ulva species. Algol Stud 138:13–26
Vidali M (2001) Bioremediation. An overview. Pure Appl Chem 73:1163–1172
Vieria R, Volesky B (2000) Biosorption: a solution to pollution? Int Microbiol 3:17–24
Volesky B (1990) Biosorption of heavy metals. CRC Press, Boca Raton
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Whitehead J, Coughanowr C, Agius J, Chrispijn J, Taylor U, Wells F (2010) State of the Derwent Estuary 2009: a review of pollution sources, loads and environmental quality data from 2003 to 2009. Department of Primary Industry, Parks, Water and Environment, Hobart, Tasmania, Australia. pp 189
Yong YS, Yong WTL, Anton A (2013) Analysis of formulae for determination of seaweed growth rate. J Appl Phycol 25:1831–1834
Young ML (1975) The transfer of 65Zn and 59Fe along a Fucus serratus (L.) → Littorina obtusata (L.) food chain. J Mar Biol Assoc UK 55:583–610
Zakeri HA, Abu Bakar L (2012) Copper-, lead- and mercury-induced changes in maximum quantum yield, chlorophyll a content and relative growth of three Malaysian green macroalgae. Malaysian J Fundament Appl Sci 9:16–21
Zbikowski R, Szefer P, Latala A (2007) Comparison of green algae Cladophora sp. and Enteromorpha sp. as potential biomonitors of chemical elements in the southern Baltic. Sci Total Environ 387:320–332
Zhou Y, Yang H, Hu H, Liu Y, Mao Y, Zhou H, Xu X, Zhang F (2006) Bioremediation potential of the macroalga Gracilaria lemaneiformis (Rhodophyta) integrated into fed fish culture in coastal waters of North China. Aquaculture 252:264–276
Zhou Q, Zhang J, Fu J, Shi J, Jiang G (2008) Biomonitoring: an appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal Chim Acta 606:135–150
Acknowledgements
The authors would like to acknowledge the Derwent Estuary Program (DEP) and Storm Bay project (FRDC project 2014/031) for providing the environmental data to the Central Laboratory of Electron Microscopy, Federal University of Santa Catarina, Florianopolis, Santa Catarina, Brazil (LCME-UFSC) for the use of their transmission electron microscope and to the Prince of Wales Bay Marina (POW) for their facilities. Special thanks are given to Lisette Robertson for her valuable support in the laboratory and to Travis Baulch and Luis Henriquez for their support on the field. This research was funded by The Holsworth Wildlife Research Endowment—Equity Trustees Charitable Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farias, D.R., Hurd, C.L., Eriksen, R.S. et al. In situ assessment of Ulva australis as a monitoring and management tool for metal pollution. J Appl Phycol 29, 2489–2502 (2017). https://doi.org/10.1007/s10811-017-1073-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1073-y