Abstract
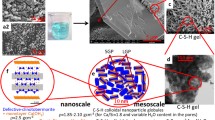
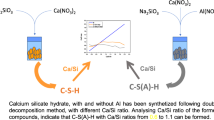
This work explores from a theoretical viewpoint the underlying growth mechanisms which govern the formation of the most important hydration product present in cementitious environments, the so called C–S–H (calcium–silicate–hydrate) gel. Aiming at identifying the basic building blocks which make up the cementitious C–S–H gel, we have performed ab-initio calculations at the Hartree Fock (HF) level. Two different growth mechanisms have been identified depending on the amount of Si and Ca ions, which naturally lead to the appearance of tobermorite-like and jennite-like nano-crystals.
Similar content being viewed by others
References
Taylor H.F. (1986) Proposed structure for calcium silicate hydrate gel. J. Am. Ceram. Soc. 69(6): 464–467
Ramachandran V.S., Beaudoin J.J. (2001) Handbook of Analytical Techniques in Concrete. William Andrew Publishing, New York
Richardson I.G., Groves G.W. (1992) Cement Concrete Res. 22, 1001–1010
Cong X., Kirkpatrick R.J. (1996) Adv. Cem. Based Mater. 3, 144–146
Nonat A., Lecoq X. (1998) The structure, stoichiometry and properties of C–S–H prepared by C3S hydration under controlled conditions. Nuclear Magnetic resonance Spectroscopy of Cement-Based Materials. Springer, Berlin, pp. 197–207
Chen J.J., Thomas J.T, Taylor H.F.W., Jennings H.M. (2004) Cement Concrete Res. 34, 1499–1519
Jönsson B., Nonat A., Labbez C., Cabane B., Wennerström H. (2005). Langmuir 21, 9211–9221
Pellenq R.J.-M., Caillol J.M., Delville A. (1997) J. Phys. Chem. B 101, 8584–8584
Delville A., Pellenq R.J.-M. (2000) Mol. Simulat. 24, 1–24
Derjaguin, B., Landau, L.D. Acta Physicochim. URSS 14, 635 (1941); Verwey, E.J.W., Overbeek, J.T.G.: Theory of the Stability of Lyophobic Colloids. Elsevier, New York (1958)
Gmira A., Zabat M., Pellenq R.J.-M., Van Damme H. (2004) Mat. struct./Concrete Sci. Eng. 37, 3–14
Faucon P., et al. (1999) J. Phys. Chem. B 103, 7796–7802
Kalinitchev A., Kirkpatrick R.J. (2002) Chem. Mater. 14, 3539–3549
Merlino S., Bonaccorsi E., Armbruster T. (2001) Eur. J. Mineral. 13, 577–590
Bonaccorsi E., x Merlino, E. J. Am. Ceram. Soc. 88(3), 505–512 (2005)
Bonaccorsi E., Merlino S., Taylor H.F.W. (2004) Cement Concrete Res. 34, 1481–1488
Brough A.R., Dobson C.M., Richardson I.G., Groves G.W. (1994) J. Mat. Sci. 29, 3926–3940
Richardson I.G. (2004) Cement Concrete Res. 34, 1733–1777
Jennings H.M. (2000) Cement Concrete Res. 30, 101–116
GAMESS – General Atomic and Molecular Electronic Structure System. Schmidt, M.W, Baldridge, K.K., Boatz, J.A., Elbert, S.T., Gordon, M.S., Jensen, J.J., Koseki, S., Matsunaga, N., Nguyen, K.A., Su., Windus, T.L., Dupuis, M., Montgomery, J.A. J. Comput. Chem. 14, 1347–1363 (1993)
Gaussian 03, Revision B.04, Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A., Vreven, Jr., T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H.M.X., Hratchian, E.P., Cross, J.B., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., Pople, J.A., Gaussian, Inc., Pittsburgh PA (2003)
Breneman C.M., Wiberg K.B. (1990) J. Comput. Chem. 11, 361–377
Parr R.G., Yang W. (1964). J. Am. Chem. Soc. 106: 4049
Mendez F., Gazquez J.L. (1994) J. Am. Chem. Soc. 116: 9298
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manzano, H., Ayuela, A. & Dolado, J.S. On the formation of cementitious C–S–H nanoparticles. J Computer-Aided Mater Des 14, 45–51 (2007). https://doi.org/10.1007/s10820-006-9030-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10820-006-9030-0