Abstract
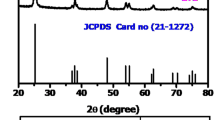
An environmentally benign synthesis method was used to prepare a nearly uniform dispersion of TiO2 nanoparticles modified by bismuth for photocatalytic purposes. The role of bismuth in the catalyst structure was evaluated using numerous methods such as XRPD, HTXRPD, TEM and HRTEM, and XPS, as well as Raman, FTIR, and UV–Vis spectroscopy. The bismuth doping significantly improved the photocatalytic performance of azo dye RB5 discoloration due to the formation of surface Bi3+ species and the abundant hydroxylation of the catalyst surface. The great advantage of this procedure lies in the low temperature preparation under ambient pressure without use of the titanium organometallic precursors. Therefore, this developed synthesis procedure could be easily adapted to the industrial scale.









Similar content being viewed by others
References
Ni M, Leung MKH, Leung DYC, Sumathy K (2007) A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew Sust Energ Rev 11:401–425
Liu G, Wang LZ, Yang HG, Cheng HM, Lu GQ (2010) Titania-based photocatalysts—crystal growth, doping and heterostructuring. J Mater Chem 20:831–843
Han F, Kambala VSR, Srinivasan M, Rajarathnam D, Naidu R (2009) Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater treatment: a review. Appl Catal A 359:25–40
Karakitsou KE, Verykios XE (1993) Effects of altervalent cation doping of TiO2 on its performance as a photocatalyst for water cleavage. J Phys Chem 97:1184–1189
Serpone N (2006) Is the band gap of pristine TiO2 narrowed by anion- and cation-doping of titanium dioxide in second-generation photocatalysts? J Phys Chem B 110:24287–24293
Oregan B, Gratzel M (1991) A low-cost, high-efficiency solar-cell based on dye-sensitized colloidal TiO2 films. Nature 353:737–740
Williams G, Seger B, Kamat PV (2008) TiO2-graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano 2:1487–1491
Wang J, Jing LQ, Xue LP, Qu YC, Fu HG (2008) Enhanced activity of bismuth-compounded TiO2 nanoparticles for photocatalytically degrading rhodamine B solution. J Hazard Mater 160:208–212
Natarajan TS, Natarajan K, Bajaj HC, Tayade RJ (2013) Enhanced photocatalytic activity of bismuth-doped TiO2 nanotubes under direct sunlight irradiation for degradation of Rhodamine B dye. J Nanopart Res 15:1669
Wu YQ, Lu GX, Li SB (2009) The doping effect of Bi on TiO2 for photocatalytic hydrogen generation and photodecolorization of Rhodamine B. J Phys Chem C 113:9950–9955
Reddy PAK, Srinivas B, Kala P, Kumari VD, Subrahmanyam M (2011) Preparation and characterization of Bi-doped TiO2 and its solar photocatalytic activity for the degradation of isoproturon herbicide. Mater Res Bull 46:1766–1771
Rengaraj S, Li XZ, Tanner PA, Pan ZF, Pang GKH (2006) Photocatalytic degradation of methylparathion—an endocrine disruptor by Bi3+-doped TiO2. J Mol Catal A 247:36–43
Yang J, Dai J, Li JT (2013) Visible-light-induced photocatalytic reduction of Cr(VI) with coupled Bi2O3/TiO2 photocatalyst and the synergistic bisphenol A oxidation. Environ Sci Pollut Res Int 20:2435–2447
Wang Y, Wang Y, Meng YL et al (2008) A highly efficient visible-light-activated photocatalyst based on bismuth- and sulfur-codoped TiO2. J Phys Chem C 112:6620–6626
Naik B, Parida KM, Behera GC (2011) Facile synthesis of Bi2O3/TiO2−x N x and its direct solar-light-driven photocatalytic selective hydroxylation of phenol. Chemcatchem 3:311–318
Li HY, Wang DJ, Wang P, Fan HM, Xie TF (2009) Synthesis and studies of the visible-light photocatalytic properties of near-monodisperse Bi-doped TiO2 nanospheres. Chem-Eur J 15:12521–12527
Ji TH, Yang F, Lv YY, Zhou JY, Sun JY (2009) Synthesis and visible-light photocatalytic activity of Bi-doped TiO2 nanobelts. Mater Lett 63:2044–2046
An’amt MN, Radiman S, Huang NM et al (2010) Sol-gel hydrothermal synthesis of bismuth-TiO2 nanocubes for dye-sensitized solar cell. Ceram Int 36:2215–2220
Xu JJ, Chen MD, Fu DG (2011) Study on highly visible light active Bi-doped TiO2 composite hollow sphere. Appl Surf Sci 257:7381–7386
Sajjad S, Leghari SAK, Chen F, Zhang JL (2010) Bismuth-doped ordered mesoporous TiO2: visible-light catalyst for simultaneous degradation of phenol and chromium. Chem-Eur J 16:13795–13804
Shamaila S, Sajjad AKL, Chen F, Zhang JL (2010) Study on highly visible light active Bi2O3 loaded ordered mesoporous titania. Appl Catal B 94:272–280
Bagwasi S, Niu YX, Nasir M, Tian BZ, Zhang JL (2013) The study of visible light active bismuth modified nitrogen doped titanium dioxide photocatalyst: role of bismuth. Appl Surf Sci 264:139–147
Cerny Z, Stengl V (2008) CZ200800139-A3 and CZ301006-B6
(2000) JCPDS PDF 2 Database, Release 50, International Centre for Diffraction Data, Newtown Square, PA
(2008) ICSD Database FIZ, Karlsruhe, Germany
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319
Barrett EP, Joyner LG, Halenda PP (1951) The determination of pore volume and area distributions in porous substances. 1. Computations from nitrogen isotherms. J Am Chem Soc 73:373–380
Wu QH (1989) Extraction of extinction coefficient of weak absorbing thin-films from special absorption. J Phys D 22:1384–1385
Roos A (1993) Use of an integrating sphere in solar-energy research. Sol Energ Mat Sol C 30:77–94
Stengl V, Houskova V, Bakardjieva S, Murafa N, Havlin V (2008) Optically transparent titanium dioxide particles incorporated in poly(hydroxyethyl methacrylate) thin layers. J Phys Chem C 112:19979–19985
Stengl V, Bakardjieva S, Murafa N (2009) Preparation and photocatalytic activity of rare earth doped TiO2 nanoparticles. Mater Chem Phys 114:217–226
Chang JA, Vithal M, Baek IC, Seok SI (2009) Morphological and phase evolution of TiO2 nanocrystals prepared from peroxotitanate complex aqueous solution: influence of acetic acid. J Solid State Chem 182:749–756
Stengl V, Matys Grygar T, Henych J, Kormunda M (2012) Hydrogen peroxide route to Sn-doped titania photocatalysts. Chem Cent J 6:113
Stengl V, Velicka J, Marikova M, Matys Grygar T (2011) New generation photocatalysts: how tungsten influences the nanostructure and photocatalytic activity of TiO2 in the UV and visible regions. ACS Appl Mater Interfaces 3:4014–4023
Chang SS, Clair B, Ruelle J et al (2009) Mesoporosity as a new parameter for understanding tension stress generation in trees. J Exp Bot 60:3023–3030
Kim KS, Winograd N (1975) Charge-transfer shake-up satellites in X-ray photoelectron-spectra of cations and anions of SrTiO3, TiO2 and Sc2O3. Chem Phys Lett 31:312–317
Guillemot F, Porte MC, Labrugere C, Baquey C (2002) Ti4+ to Ti3+ conversion of TiO2 uppermost layer by low-temperature vacuum annealing: interest for titanium biomedical applications. J Colloid Interface Sci 255:75–78
Pouilleau J, Devilliers D, Groult H, Marcus P (1997) Surface study of a titanium-based ceramic electrode material by X-ray photoelectron spectroscopy. J Mater Sci 32:5645–5651. doi:10.1023/A:1018645112465
Mathur S, Kuhn P (2006) CVD of titanium oxide coatings: comparative evaluation of then-nal and plasma assisted processes. Surf Coat Technol 201:807–814
Stefanov P, Shipochka M, Stefchev P, Raicheva Z, Lazarova V, Spassov L (2008) XPS characterization of TiO2 layers deposited on quartz plates. J Phys Conf Ser 100:012039
Wu XH, Qin W, He WD (2007) Thin bismuth oxide films prepared through the sol–gel method as photocatalyst. J Mol Catal A 261:167–171
Li Q, Liu H, Dong F, Fu M (2013) Hydrothermal formation of N-doped (BiO)2CO3 honeycomb-like microspheres photocatalysts with bismuth citrate and dicyandiamide as precursors. J Colloid Interface Sci 408:33–42
Koura N, Kohara S, Takeuchi K et al (1996) Alkali carbonates: Raman spectroscopy, ab initio calculations, and structure. J Mol Struct 382:163–169
Scheetz BE, White WB (1977) Vibrational-spectra of alkaline-earth double carbonates. Am Mineral 62:36–50
Arora AK, Rajalakshmi M, Ravindran TR, Sivasubramanian V (2007) Raman spectroscopy of optical phonon confinement in nanostructured materials. J Raman Spectrosc 38:604–617
Sahoo S, Arora AK, Sridharan V (2009) Raman line shapes of optical phonons of different symmetries in anatase TiO2 nanocrystals. J Phys Chem C 113:16927–16933
Topalian Z, Niklasson GA, Granqvist CG, Osterlund L (2009) Photo-fixation of SO2 in nanocrystalline TiO2 films prepared by reactive DC magnetron sputtering. Thin Solid Films 518:1341–1344
Serpone N, Emeline AV (2012) Semiconductor photocatalysis—past, present, and future outlook. J Phys Chem Lett 3:673–677
Konstantinou IK, Albanis TA (2004) TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations—a review. Appl Catal B 49:1–14
Stengl V, Matys Grygar T (2011) The simplest way to iodine-doped anatase for photocatalysts activated by visible light. Int J Photoenergy 2011:13. doi:10.1155/2011/685935
Acknowledgements
We acknowledge project OP VK ENVIMOD CZ.1.07/2.2.00/28.0205. JH and VS thank T. Matys Grygar for assistance with interpretation of the results and P. Bezdička and K. Šafářová for assistance with the XRD and TEM measurements.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Henych, J., Štengl, V., Kormunda, M. et al. Role of bismuth in nano-structured doped TiO2 photocatalyst prepared by environmentally benign soft synthesis. J Mater Sci 49, 3560–3571 (2014). https://doi.org/10.1007/s10853-014-8083-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8083-9